Esha Sadia Nasir
CORE - A Cell-Level Coarse-to-Fine Image Registration Engine for Multi-stain Image Alignment
Nov 12, 2025Abstract:Accurate and efficient registration of whole slide images (WSIs) is essential for high-resolution, nuclei-level analysis in multi-stained tissue slides. We propose a novel coarse-to-fine framework CORE for accurate nuclei-level registration across diverse multimodal whole-slide image (WSI) datasets. The coarse registration stage leverages prompt-based tissue mask extraction to effectively filter out artefacts and non-tissue regions, followed by global alignment using tissue morphology and ac- celerated dense feature matching with a pre-trained feature extractor. From the coarsely aligned slides, nuclei centroids are detected and subjected to fine-grained rigid registration using a custom, shape-aware point-set registration model. Finally, non-rigid alignment at the cellular level is achieved by estimating a non-linear dis- placement field using Coherent Point Drift (CPD). Our approach benefits from automatically generated nuclei that enhance the accuracy of deformable registra- tion and ensure precise nuclei-level correspondence across modalities. The pro- posed model is evaluated on three publicly available WSI registration datasets, and two private datasets. We show that CORE outperforms current state-of-the-art methods in terms of generalisability, precision, and robustness in bright-field and immunofluorescence microscopy WSIs
Nuclei & Glands Instance Segmentation in Histology Images: A Narrative Review
Aug 26, 2022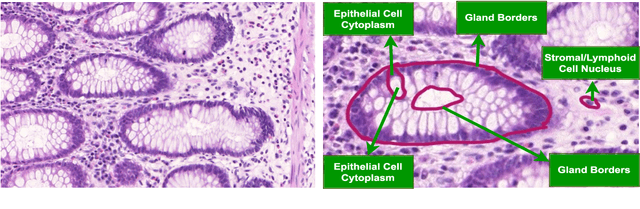
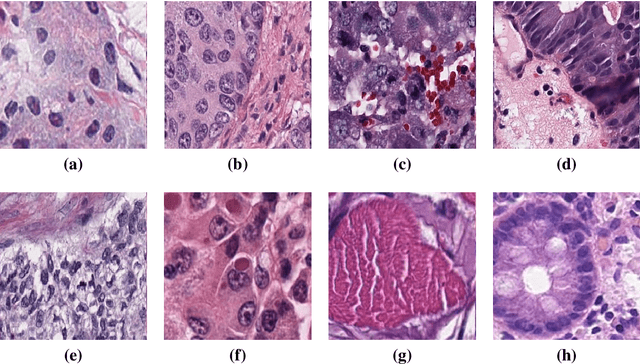
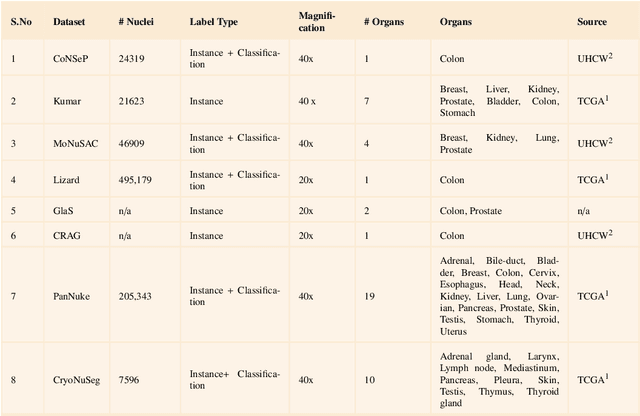
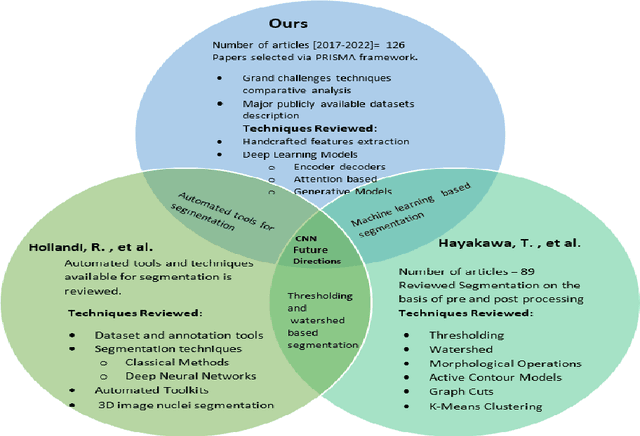
Abstract:Instance segmentation of nuclei and glands in the histology images is an important step in computational pathology workflow for cancer diagnosis, treatment planning and survival analysis. With the advent of modern hardware, the recent availability of large-scale quality public datasets and the community organized grand challenges have seen a surge in automated methods focusing on domain specific challenges, which is pivotal for technology advancements and clinical translation. In this survey, 126 papers illustrating the AI based methods for nuclei and glands instance segmentation published in the last five years (2017-2022) are deeply analyzed, the limitations of current approaches and the open challenges are discussed. Moreover, the potential future research direction is presented and the contribution of state-of-the-art methods is summarized. Further, a generalized summary of publicly available datasets and a detailed insights on the grand challenges illustrating the top performing methods specific to each challenge is also provided. Besides, we intended to give the reader current state of existing research and pointers to the future directions in developing methods that can be used in clinical practice enabling improved diagnosis, grading, prognosis, and treatment planning of cancer. To the best of our knowledge, no previous work has reviewed the instance segmentation in histology images focusing towards this direction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge