Daniel Chang
Unbiased Visual Reasoning with Controlled Visual Inputs
Dec 19, 2025Abstract:End-to-end Vision-language Models (VLMs) often answer visual questions by exploiting spurious correlations instead of causal visual evidence, and can become more shortcut-prone when fine-tuned. We introduce VISTA (Visual-Information Separation for Text-based Analysis), a modular framework that decouples perception from reasoning via an explicit information bottleneck. A frozen VLM sensor is restricted to short, objective perception queries, while a text-only LLM reasoner decomposes each question, plans queries, and aggregates visual facts in natural language. This controlled interface defines a reward-aligned environment for training unbiased visual reasoning with reinforcement learning. Instantiated with Qwen2.5-VL and Llama3.2-Vision sensors, and trained with GRPO from only 641 curated multi-step questions, VISTA significantly improves robustness to real-world spurious correlations on SpuriVerse (+16.29% with Qwen-2.5-VL-7B and +6.77% with Llama-3.2-Vision-11B), while remaining competitive on MMVP and a balanced SeedBench subset. VISTA transfers robustly across unseen VLM sensors and is able to recognize and recover from VLM perception failures. Human analysis further shows that VISTA's reasoning traces are more neutral, less reliant on spurious attributes, and more explicitly grounded in visual evidence than end-to-end VLM baselines.
Abstract: Probabilistic Prognostic Estimates of Survival in Metastatic Cancer Patients
Jul 13, 2018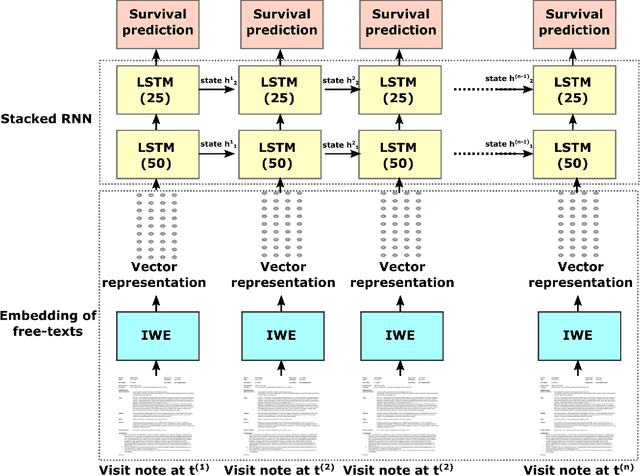
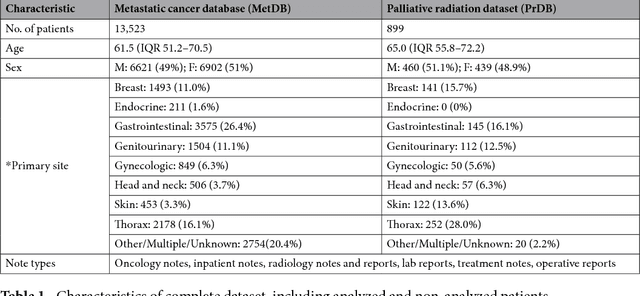
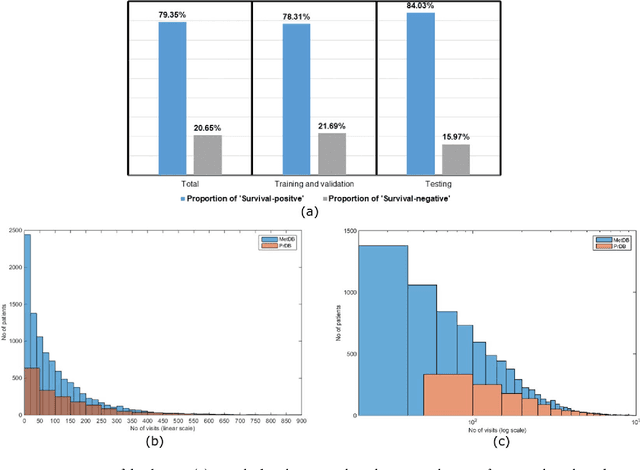
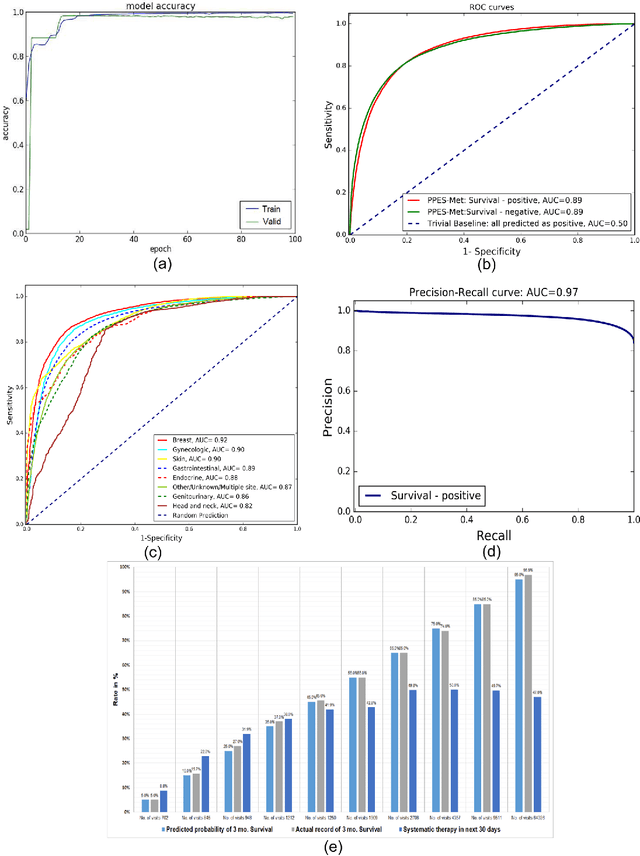
Abstract:We propose a deep learning model - Probabilistic Prognostic Estimates of Survival in Metastatic Cancer Patients (PPES-Met) for estimating short-term life expectancy (3 months) of the patients by analyzing free-text clinical notes in the electronic medical record, while maintaining the temporal visit sequence. In a single framework, we integrated semantic data mapping and neural embedding technique to produce a text processing method that extracts relevant information from heterogeneous types of clinical notes in an unsupervised manner, and we designed a recurrent neural network to model the temporal dependency of the patient visits. The model was trained on a large dataset (10,293 patients) and validated on a separated dataset (1818 patients). Our method achieved an area under the ROC curve (AUC) of 0.89. To provide explain-ability, we developed an interactive graphical tool that may improve physician understanding of the basis for the model's predictions. The high accuracy and explain-ability of the PPES-Met model may enable our model to be used as a decision support tool to personalize metastatic cancer treatment and provide valuable assistance to the physicians.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge