Chima Oluigbo
Dual-Task Graph Neural Network for Joint Seizure Onset Zone Localization and Outcome Prediction using Stereo EEG
May 29, 2025Abstract:Accurately localizing the brain regions that triggers seizures and predicting whether a patient will be seizure-free after surgery are vital for surgical planning and patient management in drug-resistant epilepsy. Stereo-electroencephalography (sEEG) delivers high-fidelity intracranial recordings that enable clinicians to precisely locate epileptogenic networks. However, the clinical identification is subjective and dependent on the expertise of the clinical team. Data driven approaches in this domain are sparse, despite the fact that sEEG offers high temporal-fidelity related to seizure dynamics that can be leveraged using graph structures ideal for imitating brain networks. In this study, we introduce a dual-task graph-neural network (GNN) framework that operates on windowed sEEG recordings to jointly predict seizure-freedom outcomes and identify seizure-onset-zone (SOZ) channels. We assemble non-overlapping 10 second windows from 51 clinical seizures spread across 20 pediatric patients, with sEEG data annotated by clinical experts. For each temporal window we construct a functional connectivity graph via thresholded Pearson correlations and extract rich node features (spectral, statistical, wavelet, Hjorth and local graph features), alongside six global graph descriptors. We optimize a combined cross-entropy loss with a tunable task-weight, and select model hyper-parameters via Optuna. Under window-level 10-fold cross-validation, the model achieves a mean graph-level accuracy of $89.31 \pm 0.0976 \%$ for seizure-freedom prediction and a node-level SOZ localization accuracy of $94.72. \pm 0.0041 \%$. For the best performing model, we ran additive and leave-one-out ablation studies to explore feature importance for graph and node-level accuracy.
Graph-Based Deep Learning on Stereo EEG for Predicting Seizure Freedom in Epilepsy Patients
Feb 21, 2025Abstract:Predicting seizure freedom is essential for tailoring epilepsy treatment. But accurate prediction remains challenging with traditional methods, especially with diverse patient populations. This study developed a deep learning-based graph neural network (GNN) model to predict seizure freedom from stereo electroencephalography (sEEG) data in patients with refractory epilepsy. We utilized high-quality sEEG data from 15 pediatric patients to train a deep learning model that can accurately predict seizure freedom outcomes and advance understanding of brain connectivity at the seizure onset zone. Our model integrates local and global connectivity using graph convolutions with multi-scale attention mechanisms to capture connections between difficult-to-study regions such as the thalamus and motor regions. The model achieved an accuracy of 92.4% in binary class analysis, 86.6% in patient-wise analysis, and 81.4% in multi-class analysis. Node and edge-level feature analysis highlighted the anterior cingulate and frontal pole regions as key contributors to seizure freedom outcomes. The nodes identified by our model were also more likely to coincide with seizure onset zones. Our findings underscore the potential of new connectivity-based deep learning models such as GNNs for enhancing the prediction of seizure freedom, predicting seizure onset zones, connectivity analysis of the brain during seizure, as well as informing AI-assisted personalized epilepsy treatment planning.
A Surgical Platform for Intracerebral Hemorrhage Robotic Evacuation (ASPIHRE): A Non-metallic MR-guided Concentric Tube Robot
Jun 20, 2022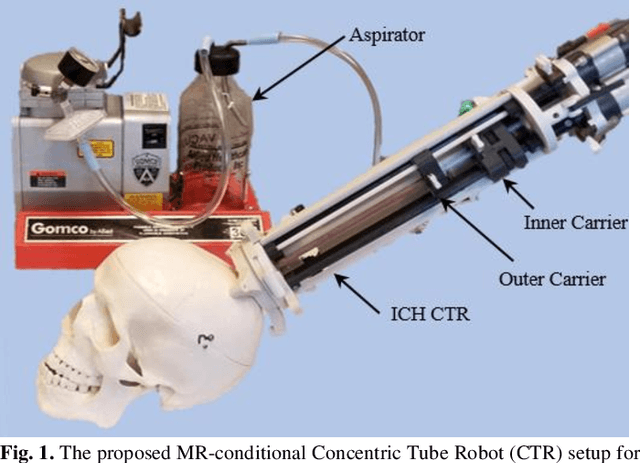
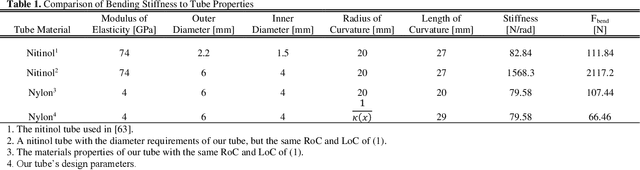
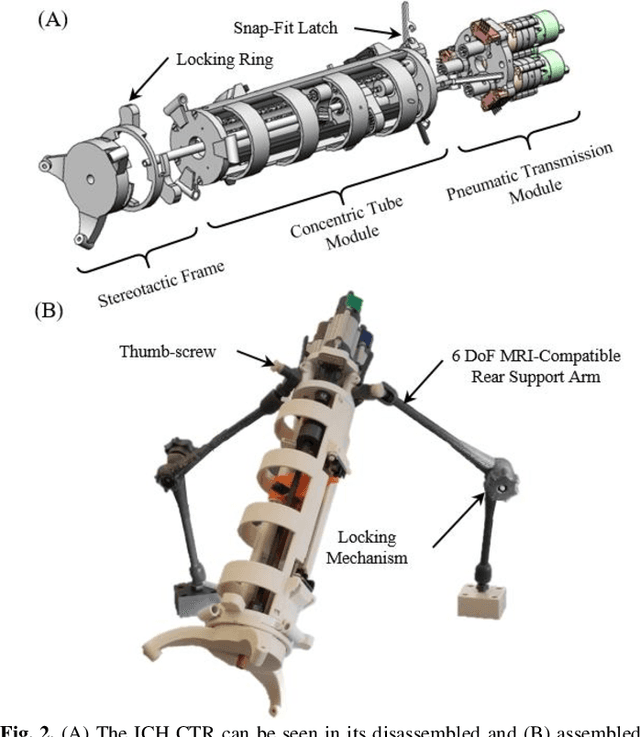
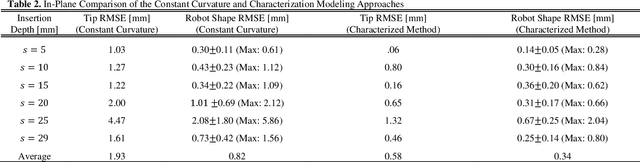
Abstract:Intracerebral hemorrhage (ICH) is the deadliest stroke sub-type, with a one-month mortality rate as high as 52%. Due to the potential cortical disruption caused by craniotomy, conservative management (watchful waiting) has historically been a common method of treatment. Minimally invasive evacuation has recently become an accepted method of treatment for patients with deep-seated hematoma 30-50 mL in volume, but proper visualization and tool dexterity remain constrained in conventional endoscopic approaches, particularly with larger hematoma volumes (> 50 mL). In this article we describe the development of ASPIHRE (A Surgical Platform for Intracerebral Hemorrhage Robotic Evacuation), the first-ever concentric tube robot that uses off-the-shelf plastic tubes for MR-guided ICH evacuation, improving tool dexterity and procedural visualization. The robot kinematics model is developed based on a calibration-based method and tube mechanics modeling, allowing the models to consider both variable curvature and torsional deflection. The MR-safe pneumatic motors are controlled using a variable gain PID algorithm producing a rotational accuracy of 0.317 +/- 0.3 degrees. The hardware and theoretical models are validated in a series of systematic bench-top and MRI experiments resulting in positional accuracy of the tube tip of 1.39 +\- 0.54 mm. Following validation of targeting accuracy, the evacuation efficacy of the robot was tested in an MR-guided phantom clot evacuation experiment. The robot was able to evacuate an initially 38.36 mL clot in 5 minutes, leaving a residual hematoma of 8.14 mL, well below the 15 mL guideline suggesting good post-ICH evacuation clinical outcomes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge