Yunchuan Kong
forgeNet: A graph deep neural network model using tree-based ensemble classifiers for feature extraction
May 23, 2019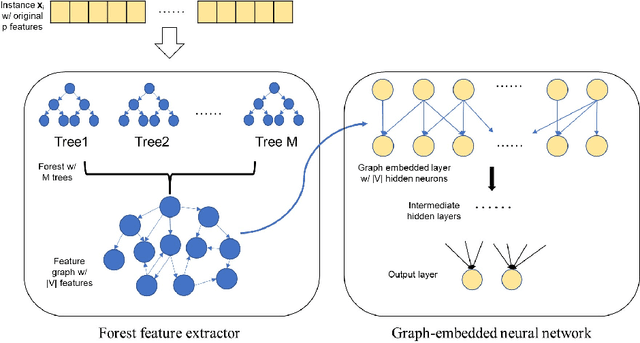

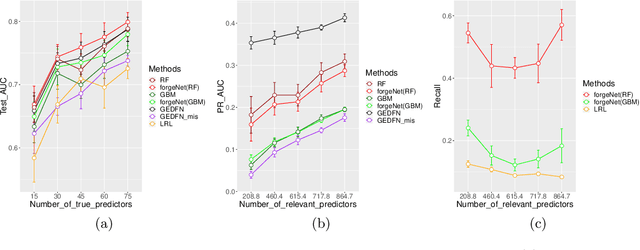

Abstract:A unique challenge in predictive model building for omics data has been the small number of samples $(n)$ versus the large amount of features $(p)$. This "$n\ll p$" property brings difficulties for disease outcome classification using deep learning techniques. Sparse learning by incorporating external gene network information such as the graph-embedded deep feedforward network (GEDFN) model has been a solution to this issue. However, such methods require an existing feature graph, and potential mis-specification of the feature graph can be harmful on classification and feature selection. To address this limitation and develop a robust classification model without relying on external knowledge, we propose a \underline{for}est \underline{g}raph-\underline{e}mbedded deep feedforward \underline{net}work (forgeNet) model, to integrate the GEDFN architecture with a forest feature graph extractor, so that the feature graph can be learned in a supervised manner and specifically constructed for a given prediction task. To validate the method's capability, we experimented the forgeNet model with both synthetic and real datasets. The resulting high classification accuracy suggests that the method is a valuable addition to sparse deep learning models for omics data.
A graph-embedded deep feedforward network for disease outcome classification and feature selection using gene expression data
Feb 12, 2018



Abstract:Gene expression data represents a unique challenge in predictive model building, because of the small number of samples $(n)$ compared to the huge amount of features $(p)$. This "$n<<p$" property has hampered application of deep learning techniques for disease outcome classification. Sparse learning by incorporating external gene network information could be a potential solution to this issue. Still, the problem is very challenging because (1) there are tens of thousands of features and only hundreds of training samples, (2) the scale-free structure of the gene network is unfriendly to the setup of convolutional neural networks. To address these issues and build a robust classification model, we propose the Graph-Embedded Deep Feedforward Networks (GEDFN), to integrate external relational information of features into the deep neural network architecture. The method is able to achieve sparse connection between network layers to prevent overfitting. To validate the method's capability, we conducted both simulation experiments and a real data analysis using a breast cancer RNA-seq dataset from The Cancer Genome Atlas (TCGA). The resulting high classification accuracy and easily interpretable feature selection results suggest the method is a useful addition to the current classification models and feature selection procedures. The method is available at https://github.com/yunchuankong/NetworkNeuralNetwork.
A Bayesian Method for Joint Clustering of Vectorial Data and Network Data
Oct 24, 2017
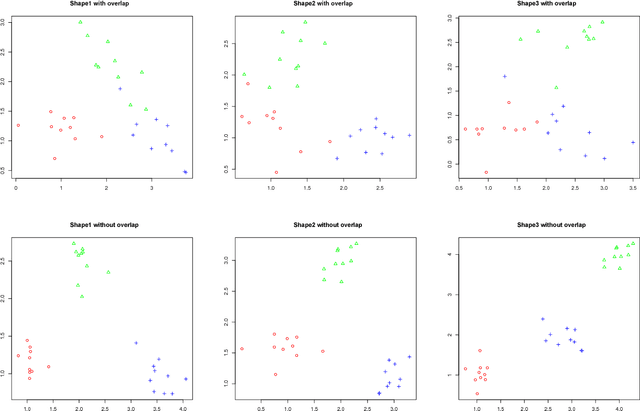
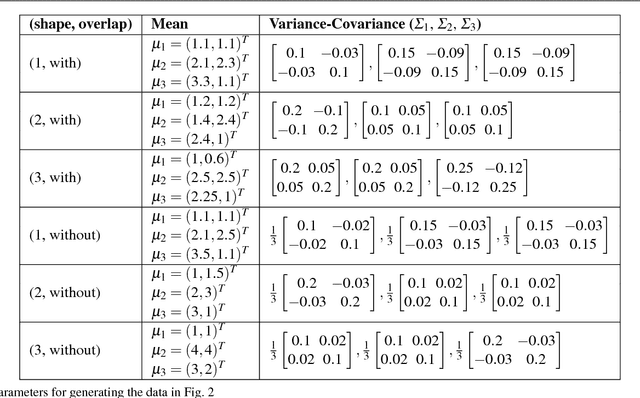
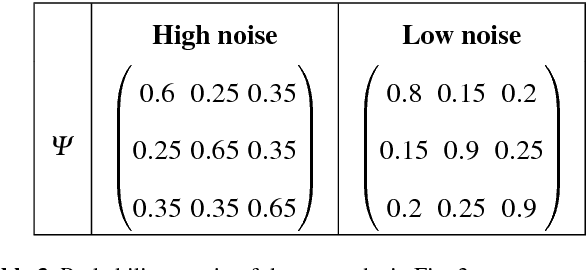
Abstract:We present a new model-based integrative method for clustering objects given both vectorial data, which describes the feature of each object, and network data, which indicates the similarity of connected objects. The proposed general model is able to cluster the two types of data simultaneously within one integrative probabilistic model, while traditional methods can only handle one data type or depend on transforming one data type to another. Bayesian inference of the clustering is conducted based on a Markov chain Monte Carlo algorithm. A special case of the general model combining the Gaussian mixture model and the stochastic block model is extensively studied. We used both synthetic data and real data to evaluate this new method and compare it with alternative methods. The results show that our simultaneous clustering method performs much better. This improvement is due to the power of the model-based probabilistic approach for efficiently integrating information.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge