Xiao-Yun Zhou
Estimation of Tissue Oxygen Saturation from RGB Images based on Pixel-level Image Translation
Apr 19, 2018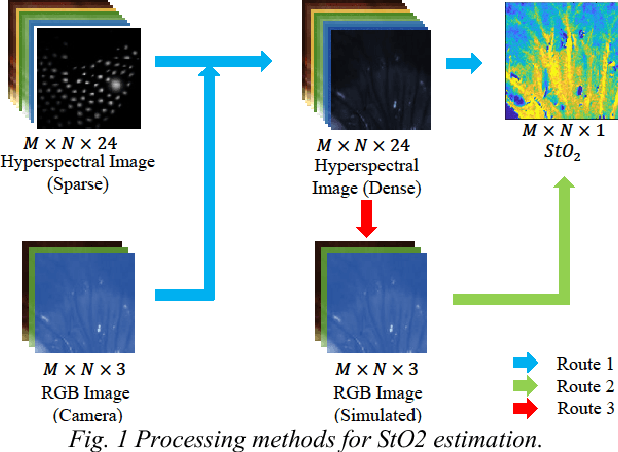
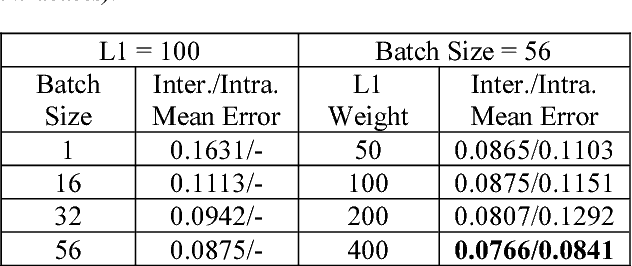
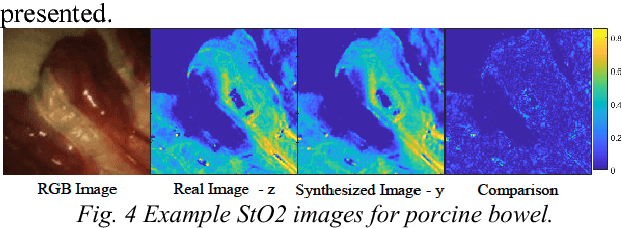
Abstract:Intra-operative measurement of tissue oxygen saturation (StO2) has been widely explored by pulse oximetry or hyperspectral imaging (HSI) to assess the function and viability of tissue. In this paper we propose a pixel- level image-to-image translation approach based on conditional Generative Adversarial Networks (cGAN) to estimate tissue oxygen saturation (StO2) directly from RGB images. The real-time performance and non-reliance on additional hardware, enable a seamless integration of the proposed method into surgical and diagnostic workflows with standard endoscope systems. For validation, RGB images and StO2 ground truth were simulated and estimated from HSI images collected by a liquid crystal tuneable filter (LCTF) endoscope for three tissue types (porcine bowel, lamb uterus and rabbit uterus). The result show that the proposed method can achieve visually identical images with comparable accuracy.
Abdominal Aortic Aneurysm Segmentation with a Small Number of Training Subjects
Apr 09, 2018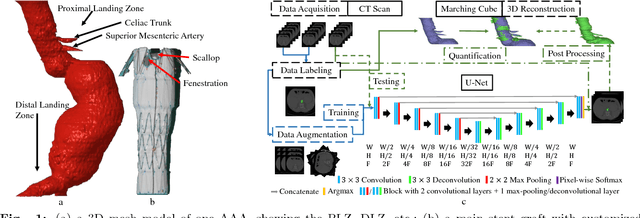
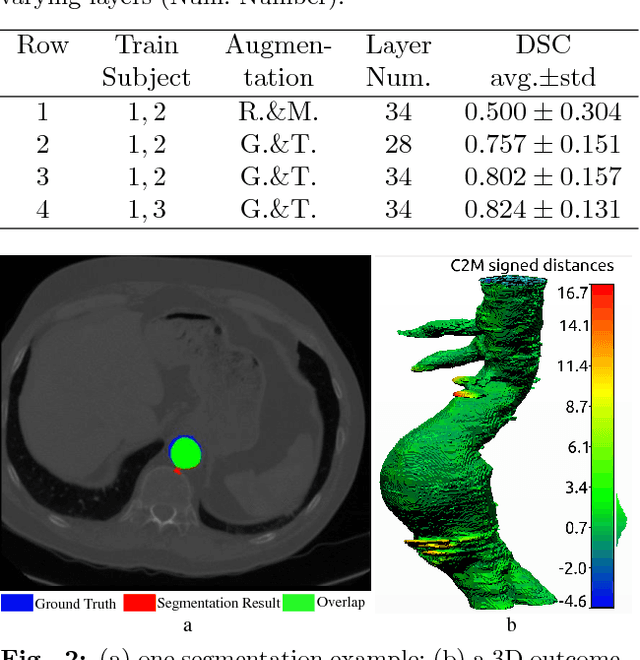
Abstract:Pre-operative Abdominal Aortic Aneurysm (AAA) 3D shape is critical for customized stent-graft design in Fenestrated Endovascular Aortic Repair (FEVAR). Traditional segmentation approaches implement expert-designed feature extractors while recent deep neural networks extract features automatically with multiple non-linear modules. Usually, a large training dataset is essential for applying deep learning on AAA segmentation. In this paper, the AAA was segmented using U-net with a small number (two) of training subjects. Firstly, Computed Tomography Angiography (CTA) slices were augmented with gray value variation and translation to avoid the overfitting caused by the small number of training subjects. Then, U-net was trained to segment the AAA. Dice Similarity Coefficients (DSCs) over 0.8 were achieved on the testing subjects. The PLZ, DLZ and aortic branches are all reconstructed reasonably, which will facilitate stent graft customization and help shape instantiation for intra-operative surgery navigation in FEVAR.
Real-time 3D Shape Instantiation from Single Fluoroscopy Projection for Fenestrated Stent Graft Deployment
Jan 08, 2018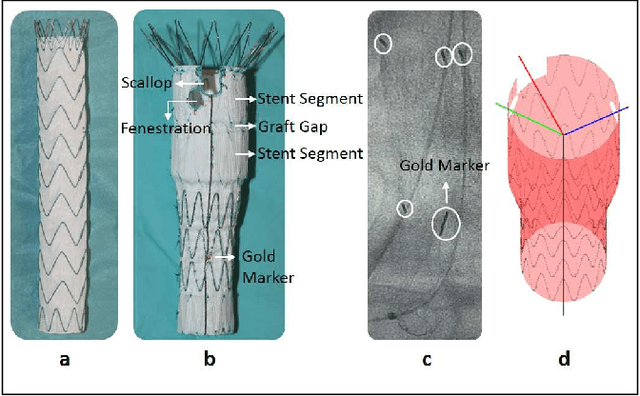
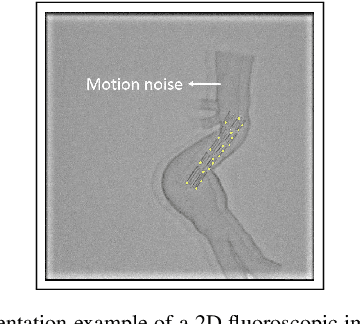
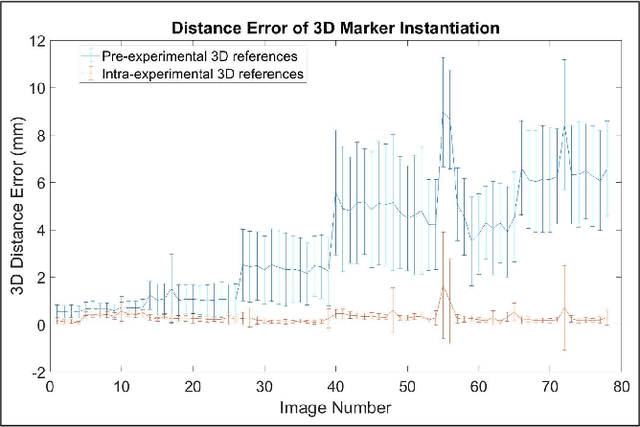
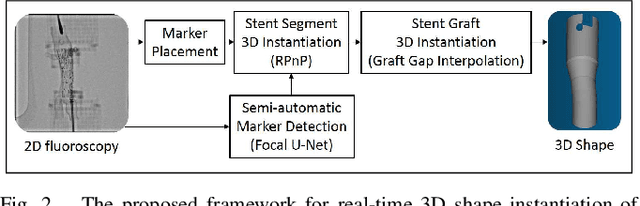
Abstract:Robot-assisted deployment of fenestrated stent grafts in Fenestrated Endovascular Aortic Repair (FEVAR) requires accurate geometrical alignment. Currently, this process is guided by 2D fluoroscopy, which is uninformative and error prone. In this paper, a real-time framework is proposed to instantiate the 3D shape of a fenestrated stent graft based on only a single low-dose 2D fluoroscopic image. Firstly, the fenestrated stent graft was placed with markers. Secondly, the 3D pose of each stent segment was instantiated by the RPnP (Robust Perspective-n-Point) method. Thirdly, the 3D shape of the whole stent graft was instantiated via graft gap interpolation. Focal-Unet was proposed to segment the markers from 2D fluoroscopic images to achieve semi-automatic marker detection. The proposed framework was validated on five patient-specific 3D printed phantoms of aortic aneurysms and three stent grafts with new marker placements, showing an average distance error of 1-3mm and an average angle error of 4 degree.
* 7 pages, 10 figures
A Real-time and Registration-free Framework for Dynamic Shape Instantiation
Dec 30, 2017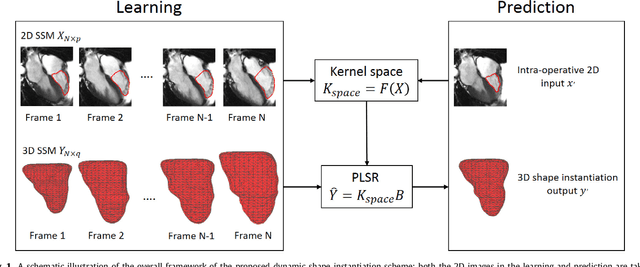
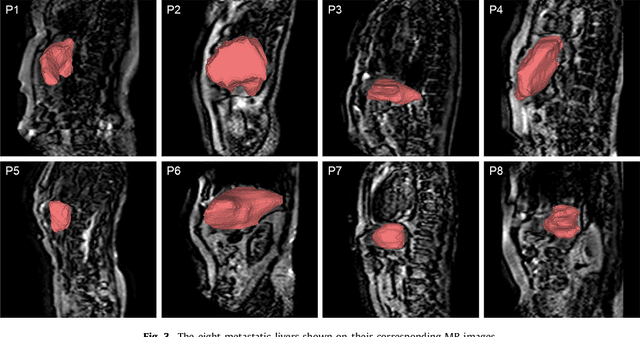
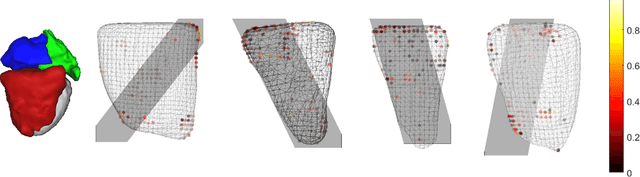
Abstract:Real-time 3D navigation during minimally invasive procedures is an essential yet challenging task, especially when considerable tissue motion is involved. To balance image acquisition speed and resolution, only 2D images or low-resolution 3D volumes can be used clinically. In this paper, a real-time and registration-free framework for dynamic shape instantiation, generalizable to multiple anatomical applications, is proposed to instantiate high-resolution 3D shapes of an organ from a single 2D image intra-operatively. Firstly, an approximate optimal scan plane was determined by analyzing the pre-operative 3D statistical shape model (SSM) of the anatomy with sparse principal component analysis (SPCA) and considering practical constraints . Secondly, kernel partial least squares regression (KPLSR) was used to learn the relationship between the pre-operative 3D SSM and a synchronized 2D SSM constructed from 2D images obtained at the approximate optimal scan plane. Finally, the derived relationship was applied to the new intra-operative 2D image obtained at the same scan plane to predict the high-resolution 3D shape intra-operatively. A major feature of the proposed framework is that no extra registration between the pre-operative 3D SSM and the synchronized 2D SSM is required. Detailed validation was performed on studies including the liver and right ventricle (RV) of the heart. The derived results (mean accuracy of 2.19mm on patients and computation speed of 1ms) demonstrate its potential clinical value for real-time, high-resolution, dynamic and 3D interventional guidance.
* 35 Pages, 11 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge