Peter Baker
Autonomous battery research: Principles of heuristic operando experimentation
Dec 29, 2025Abstract:Unravelling the complex processes governing battery degradation is critical to the energy transition, yet the efficacy of operando characterisation is severely constrained by a lack of Reliability, Representativeness, and Reproducibility (the 3Rs). Current methods rely on bespoke hardware and passive, pre-programmed methodologies that are ill-equipped to capture stochastic failure events. Here, using the Rutherford Appleton Laboratory's multi-modal toolkit as a case study, we expose the systemic inability of conventional experiments to capture transient phenomena like dendrite initiation. To address this, we propose Heuristic Operando experiments: a framework where an AI pilot leverages physics-based digital twins to actively steer the beamline to predict and deterministically capture these rare events. Distinct from uncertainty-driven active learning, this proactive search anticipates failure precursors, redefining experimental efficiency via an entropy-based metric that prioritises scientific insight per photon, neutron, or muon. By focusing measurements only on mechanistically decisive moments, this framework simultaneously mitigates beam damage and drastically reduces data redundancy. When integrated with FAIR data principles, this approach serves as a blueprint for the trusted autonomous battery laboratories of the future.
Bayesian Network Based Risk and Sensitivity Analysis for Production Process Stability Control
Sep 10, 2019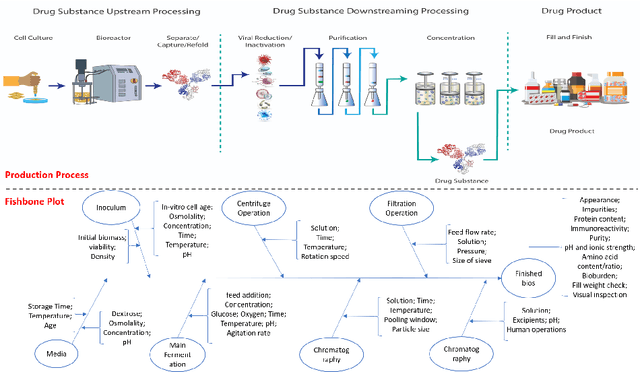
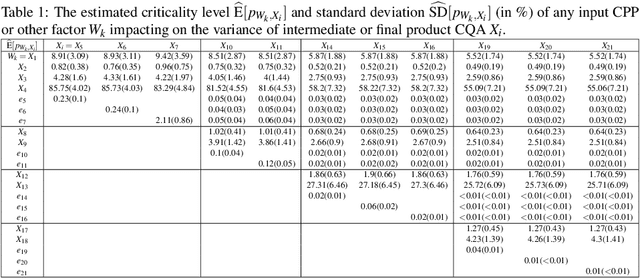
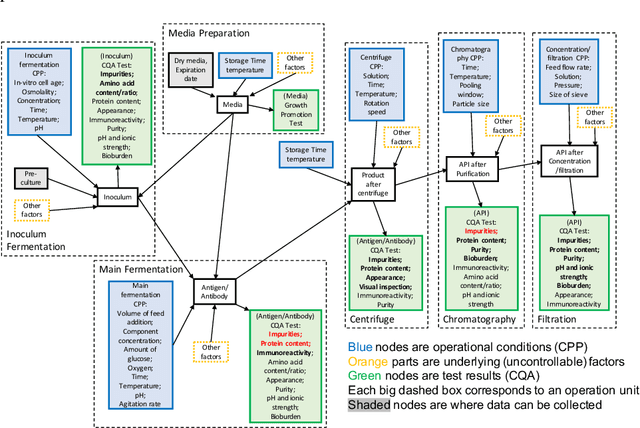
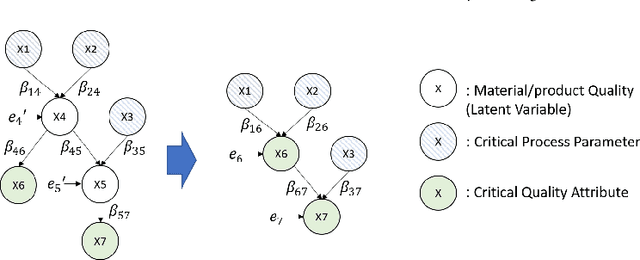
Abstract:The biomanufacturing industry is growing rapidly and becoming one of the key drivers of personalized medicine and life science. However, biopharmaceutical production faces critical challenges, including complexity, high variability, long lead time and rapid changes in technologies, processes, and regulatory environment. Driven by these challenges, we explore the bio-technology domain knowledge and propose a rigorous risk and sensitivity analysis framework for biomanufacturing innovation. Built on the causal relationships of raw material quality attributes, production process, and bio-drug properties in safety and efficacy, we develop a Bayesian Network (BN) to model the complex probabilistic interdependence between process parameters and quality attributes of raw materials/in-process materials/drug substance. It integrates various sources of data and leads to an interpretable probabilistic knowledge graph of the end-to-end production process. Then, we introduce a systematic risk analysis to assess the criticality of process parameters and quality attributes. The complex production processes often involve many process parameters and quality attributes impacting the product quality variability. However, the real-world (batch) data are often limited, especially for customized and personalized bio-drugs. We propose uncertainty quantification and sensitivity analysis to analyze the impact of model risk. Given very limited process data, the empirical results show that we can provide reliable and interpretable risk and sensitivity analysis. Thus, the proposed framework can provide the science- and risk-based guidance on the process monitoring, data collection, and process parameters specifications to facilitate the production process learning and stability control.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge