Meng Zhu
AdamX: An Adam improvement algorithm based on a novel exponential decay mechanism for the second-order moment estimate
Nov 19, 2025



Abstract:Since the 21st century, artificial intelligence has been leading a new round of industrial revolution. Under the training framework, the optimization algorithm aims to stably converge high-dimensional optimization to local and even global minima. Entering the era of large language models, although the scale of model parameters and data has increased, Adam remains the mainstream optimization algorithm. However, compared with stochastic gradient descent (SGD) based optimization algorithms, Adam is more likely to converge to non-flat minima. To address this issue, the AdamX algorithm is proposed. Its core innovation lies in the proposition of a novel type of second-order moment estimation exponential decay rate, which gradually weakens the learning step correction strength as training progresses, and degrades to SGD in the stable training period, thereby improving the stability of training in the stable period and possibly enhancing generalization ability. Experimental results show that our second-order moment estimation exponential decay rate is better than the current second-order moment estimation exponential decay rate, and AdamX can stably outperform Adam and its variants in terms of performance. Our code is open-sourced at https://github.com/mengzhu0308/AdamX.
Stain-free Detection of Embryo Polarization using Deep Learning
Nov 08, 2021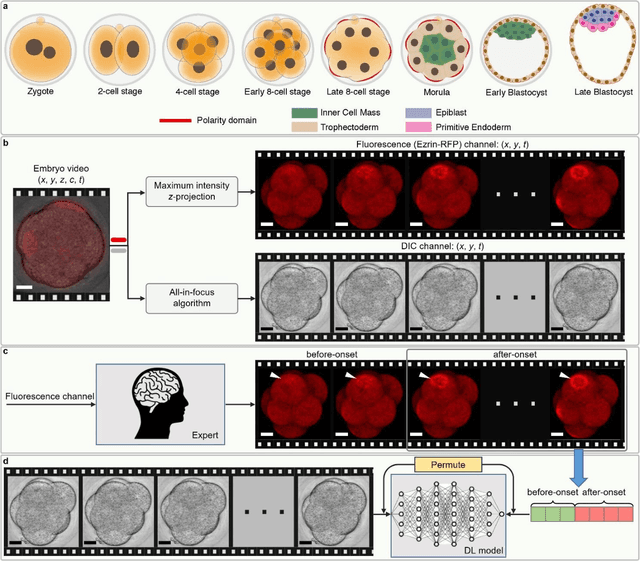
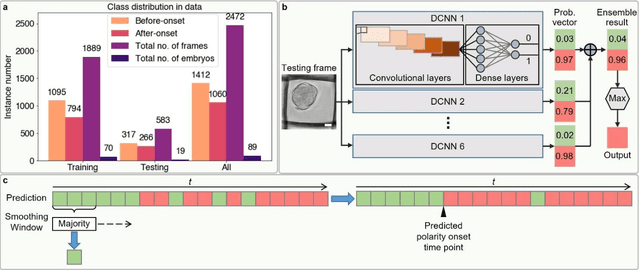
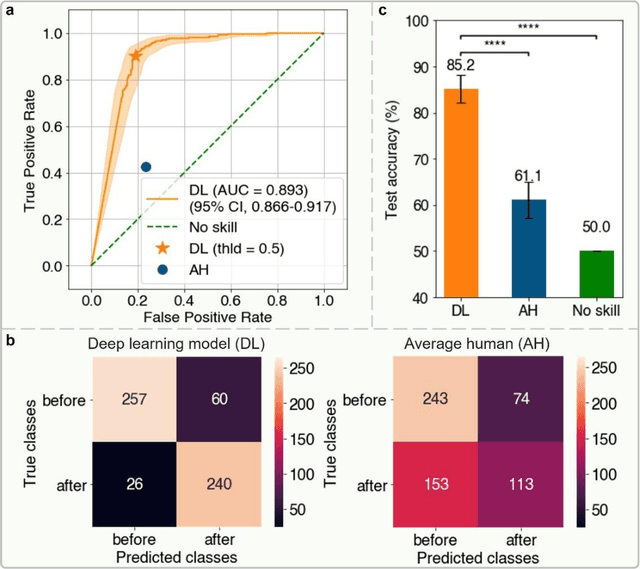
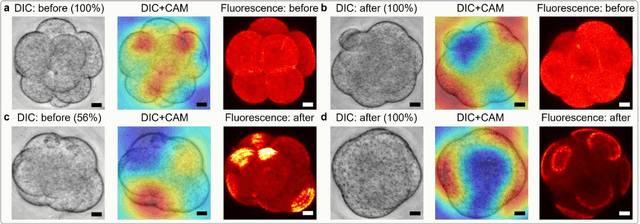
Abstract:Polarization of the mammalian embryo at the right developmental time is critical for its development to term and would be valuable in assessing the potential of human embryos. However, tracking polarization requires invasive fluorescence staining, impermissible in the in vitro fertilization clinic. Here, we report the use of artificial intelligence to detect polarization from unstained time-lapse movies of mouse embryos. We assembled a dataset of bright-field movie frames from 8-cell-stage embryos, side-by-side with corresponding images of fluorescent markers of cell polarization. We then used an ensemble learning model to detect whether any bright-field frame showed an embryo before or after onset of polarization. Our resulting model has an accuracy of 85% for detecting polarization, significantly outperforming human volunteers trained on the same data (61% accuracy). We discovered that our self-learning model focuses upon the angle between cells as one known cue for compaction, which precedes polarization, but it outperforms the use of this cue alone. By compressing three-dimensional time-lapsed image data into two-dimensions, we are able to reduce data to an easily manageable size for deep learning processing. In conclusion, we describe a method for detecting a key developmental feature of embryo development that avoids clinically impermissible fluorescence staining.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge