Lara Cavinato
DISARM++: Beyond scanner-free harmonization
May 06, 2025



Abstract:Harmonization of T1-weighted MR images across different scanners is crucial for ensuring consistency in neuroimaging studies. This study introduces a novel approach to direct image harmonization, moving beyond feature standardization to ensure that extracted features remain inherently reliable for downstream analysis. Our method enables image transfer in two ways: (1) mapping images to a scanner-free space for uniform appearance across all scanners, and (2) transforming images into the domain of a specific scanner used in model training, embedding its unique characteristics. Our approach presents strong generalization capability, even for unseen scanners not included in the training phase. We validated our method using MR images from diverse cohorts, including healthy controls, traveling subjects, and individuals with Alzheimer's disease (AD). The model's effectiveness is tested in multiple applications, such as brain age prediction (R2 = 0.60 \pm 0.05), biomarker extraction, AD classification (Test Accuracy = 0.86 \pm 0.03), and diagnosis prediction (AUC = 0.95). In all cases, our harmonization technique outperforms state-of-the-art methods, showing improvements in both reliability and predictive accuracy. Moreover, our approach eliminates the need for extensive preprocessing steps, such as skull-stripping, which can introduce errors by misclassifying brain and non-brain structures. This makes our method particularly suitable for applications that require full-head analysis, including research on head trauma and cranial deformities. Additionally, our harmonization model does not require retraining for new datasets, allowing smooth integration into various neuroimaging workflows. By ensuring scanner-invariant image quality, our approach provides a robust and efficient solution for improving neuroimaging studies across diverse settings. The code is available at this link.
Imaging-based representation and stratification of intra-tumor Heterogeneity via tree-edit distance
Aug 09, 2022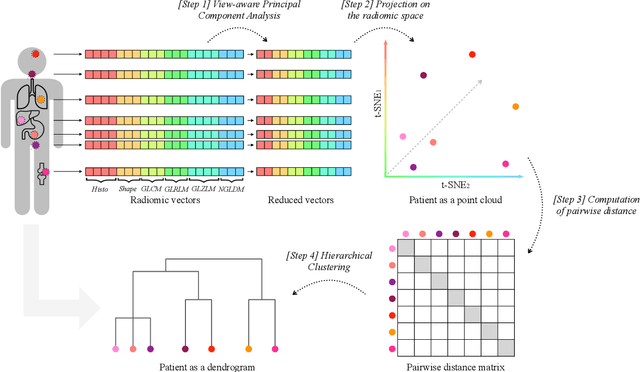
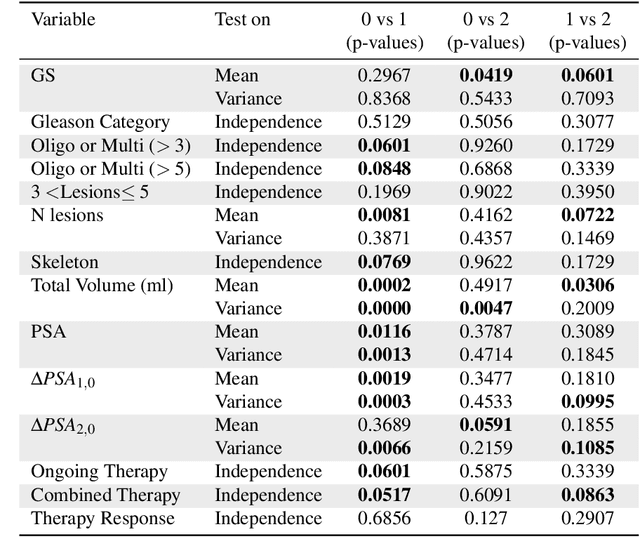
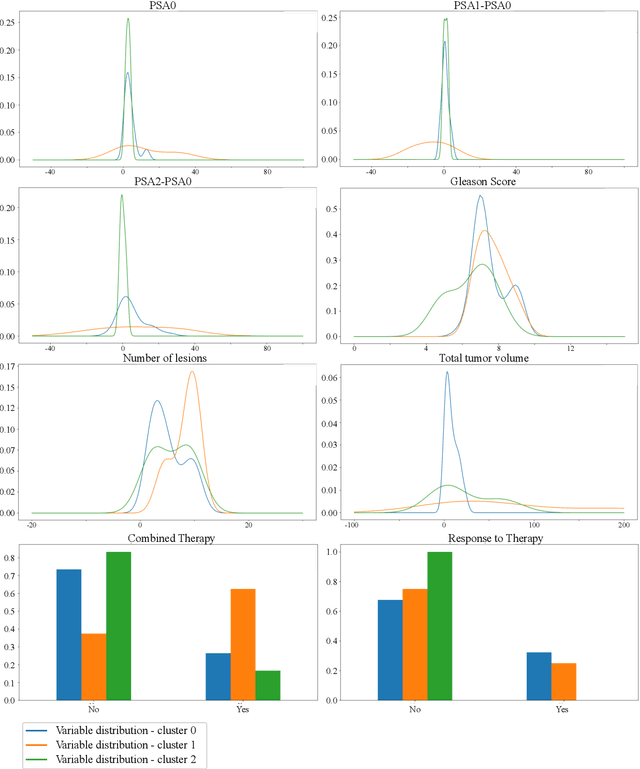
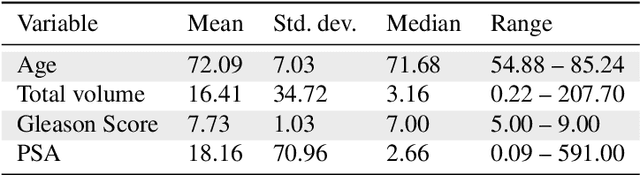
Abstract:Personalized medicine is the future of medical practice. In oncology, tumor heterogeneity assessment represents a pivotal step for effective treatment planning and prognosis prediction. Despite new procedures for DNA sequencing and analysis, non-invasive methods for tumor characterization are needed to impact on daily routine. On purpose, imaging texture analysis is rapidly scaling, holding the promise to surrogate histopathological assessment of tumor lesions. In this work, we propose a tree-based representation strategy for describing intra-tumor heterogeneity of patients affected by metastatic cancer. We leverage radiomics information extracted from PET/CT imaging and we provide an exhaustive and easily readable summary of the disease spreading. We exploit this novel patient representation to perform cancer subtyping according to hierarchical clustering technique. To this purpose, a new heterogeneity-based distance between trees is defined and applied to a case study of Prostate Cancer (PCa). Clusters interpretation is explored in terms of concordance with severity status, tumor burden and biological characteristics. Results are promising, as the proposed method outperforms current literature approaches. Ultimately, the proposed methods draws a general analysis framework that would allow to extract knowledge from daily acquired imaging data of patients and provide insights for effective treatment planning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge