John S. Duncan
Department of Clinical and Experimental Epilepsy, UCL Queen Square Institute of Neurology, National Hospital for Neurology and Neurosurgery, Queen Square, London, UK
A self-supervised learning strategy for postoperative brain cavity segmentation simulating resections
May 24, 2021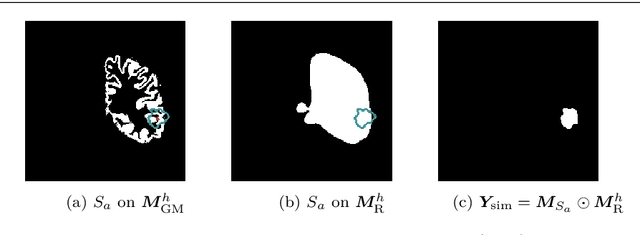
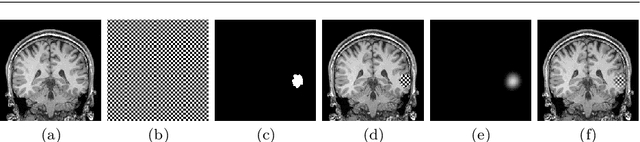
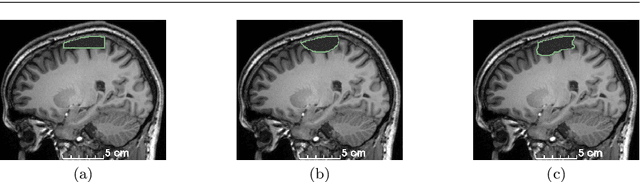
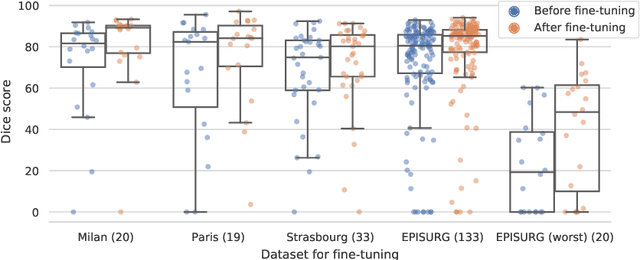
Abstract:Accurate segmentation of brain resection cavities (RCs) aids in postoperative analysis and determining follow-up treatment. Convolutional neural networks (CNNs) are the state-of-the-art image segmentation technique, but require large annotated datasets for training. Annotation of 3D medical images is time-consuming, requires highly-trained raters, and may suffer from high inter-rater variability. Self-supervised learning strategies can leverage unlabeled data for training. We developed an algorithm to simulate resections from preoperative magnetic resonance images (MRIs). We performed self-supervised training of a 3D CNN for RC segmentation using our simulation method. We curated EPISURG, a dataset comprising 430 postoperative and 268 preoperative MRIs from 430 refractory epilepsy patients who underwent resective neurosurgery. We fine-tuned our model on three small annotated datasets from different institutions and on the annotated images in EPISURG, comprising 20, 33, 19 and 133 subjects. The model trained on data with simulated resections obtained median (interquartile range) Dice score coefficients (DSCs) of 81.7 (16.4), 82.4 (36.4), 74.9 (24.2) and 80.5 (18.7) for each of the four datasets. After fine-tuning, DSCs were 89.2 (13.3), 84.1 (19.8), 80.2 (20.1) and 85.2 (10.8). For comparison, inter-rater agreement between human annotators from our previous study was 84.0 (9.9). We present a self-supervised learning strategy for 3D CNNs using simulated RCs to accurately segment real RCs on postoperative MRI. Our method generalizes well to data from different institutions, pathologies and modalities. Source code, segmentation models and the EPISURG dataset are available at https://github.com/fepegar/ressegijcars .
Simulation of Brain Resection for Cavity Segmentation Using Self-Supervised and Semi-Supervised Learning
Jun 28, 2020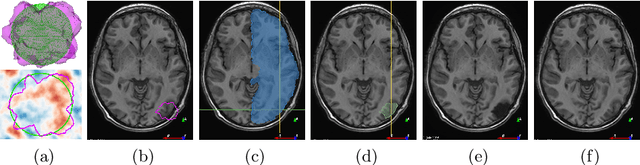
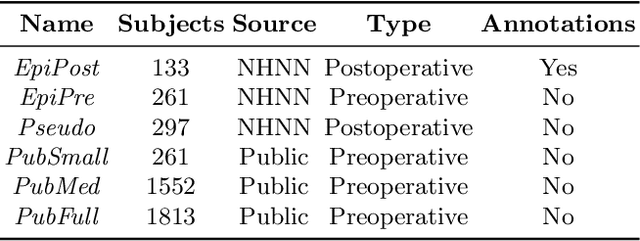
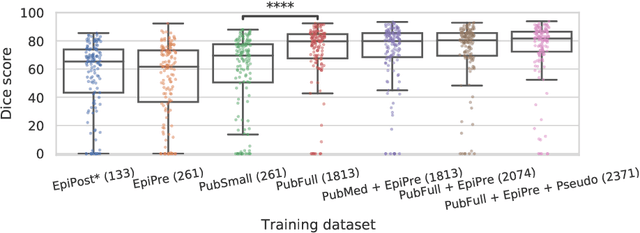
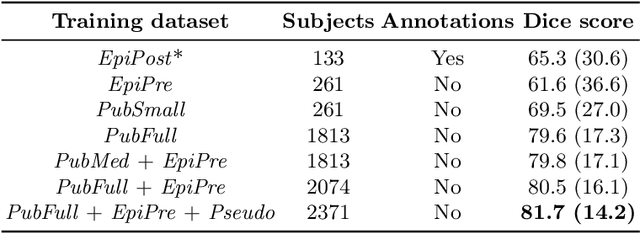
Abstract:Resective surgery may be curative for drug-resistant focal epilepsy, but only 40% to 70% of patients achieve seizure freedom after surgery. Retrospective quantitative analysis could elucidate patterns in resected structures and patient outcomes to improve resective surgery. However, the resection cavity must first be segmented on the postoperative MR image. Convolutional neural networks (CNNs) are the state-of-the-art image segmentation technique, but require large amounts of annotated data for training. Annotation of medical images is a time-consuming process requiring highly-trained raters, and often suffering from high inter-rater variability. Self-supervised learning can be used to generate training instances from unlabeled data. We developed an algorithm to simulate resections on preoperative MR images. We curated a new dataset, EPISURG, comprising 431 postoperative and 269 preoperative MR images from 431 patients who underwent resective surgery. In addition to EPISURG, we used three public datasets comprising 1813 preoperative MR images for training. We trained a 3D CNN on artificially resected images created on the fly during training, using images from 1) EPISURG, 2) public datasets and 3) both. To evaluate trained models, we calculate Dice score (DSC) between model segmentations and 200 manual annotations performed by three human raters. The model trained on data with manual annotations obtained a median (interquartile range) DSC of 65.3 (30.6). The DSC of our best-performing model, trained with no manual annotations, is 81.7 (14.2). For comparison, inter-rater agreement between human annotators was 84.0 (9.9). We demonstrate a training method for CNNs using simulated resection cavities that can accurately segment real resection cavities, without manual annotations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge