Hongjie Jiang
Risk Awareness Injection: Calibrating Vision-Language Models for Safety without Compromising Utility
Feb 03, 2026Abstract:Vision language models (VLMs) extend the reasoning capabilities of large language models (LLMs) to cross-modal settings, yet remain highly vulnerable to multimodal jailbreak attacks. Existing defenses predominantly rely on safety fine-tuning or aggressive token manipulations, incurring substantial training costs or significantly degrading utility. Recent research shows that LLMs inherently recognize unsafe content in text, and the incorporation of visual inputs in VLMs frequently dilutes risk-related signals. Motivated by this, we propose Risk Awareness Injection (RAI), a lightweight and training-free framework for safety calibration that restores LLM-like risk recognition by amplifying unsafe signals in VLMs. Specifically, RAI constructs an Unsafe Prototype Subspace from language embeddings and performs targeted modulation on selected high-risk visual tokens, explicitly activating safety-critical signals within the cross-modal feature space. This modulation restores the model's LLM-like ability to detect unsafe content from visual inputs, while preserving the semantic integrity of original tokens for cross-modal reasoning. Extensive experiments across multiple jailbreak and utility benchmarks demonstrate that RAI substantially reduces attack success rate without compromising task performance.
Brain Network Analysis Based on Fine-tuned Self-supervised Model for Brain Disease Diagnosis
Jun 13, 2025Abstract:Functional brain network analysis has become an indispensable tool for brain disease analysis. It is profoundly impacted by deep learning methods, which can characterize complex connections between ROIs. However, the research on foundation models of brain network is limited and constrained to a single dimension, which restricts their extensive application in neuroscience. In this study, we propose a fine-tuned brain network model for brain disease diagnosis. It expands brain region representations across multiple dimensions based on the original brain network model, thereby enhancing its generalizability. Our model consists of two key modules: (1)an adapter module that expands brain region features across different dimensions. (2)a fine-tuned foundation brain network model, based on self-supervised learning and pre-trained on fMRI data from thousands of participants. Specifically, its transformer block is able to effectively extract brain region features and compute the inter-region associations. Moreover, we derive a compact latent representation of the brain network for brain disease diagnosis. Our downstream experiments in this study demonstrate that the proposed model achieves superior performance in brain disease diagnosis, which potentially offers a promising approach in brain network analysis research.
A Pilot Study on the Comparison of Prefrontal Cortex Activities of Robotic Therapies on Elderly with Mild Cognitive Impairment
May 04, 2024



Abstract:Demographic shifts have led to an increase in mild cognitive impairment (MCI), and this study investigates the effects of cognitive training (CT) and reminiscence therapy (RT) conducted by humans or socially assistive robots (SARs) on prefrontal cortex activation in elderly individuals with MCI, aiming to determine the most effective therapy-modality combination for promoting cognitive function. This pilot study employs a randomized control trial (RCT) design. Additionally, the study explores the efficacy of Reminiscence Therapy (RT) in comparison to Cognitive Training (CT). Eight MCI subjects, with a mean age of 70.125 years, were randomly assigned to ``human-led'' or ``SAR-led'' groups. Utilizing Functional Near-infrared Spectroscopy (fNIRS) to measure oxy-hemoglobin concentration changes in the dorsolateral prefrontal cortex (DLPFC), the study found no significant differences in the effects of human-led and SAR-led cognitive training on DLPFC activation. However, distinct patterns emerged in memory encoding and retrieval phases between RT and CT, shedding light on the impacts of these interventions on brain activation in the context of MCI.
Dynamic Ensemble Bayesian Filter for Robust Control of a Human Brain-machine Interface
Apr 22, 2022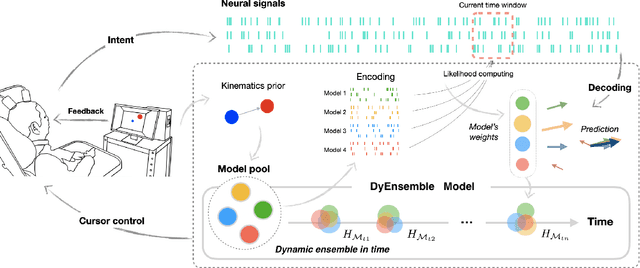
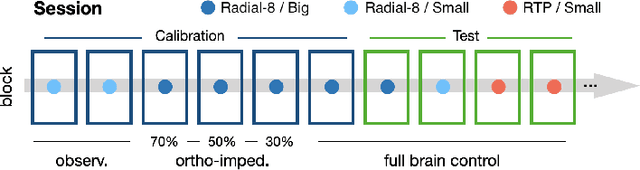
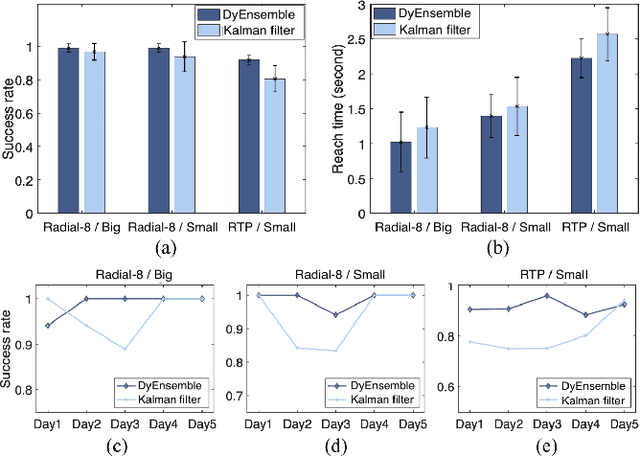
Abstract:Objective: Brain-machine interfaces (BMIs) aim to provide direct brain control of devices such as prostheses and computer cursors, which have demonstrated great potential for mobility restoration. One major limitation of current BMIs lies in the unstable performance in online control due to the variability of neural signals, which seriously hinders the clinical availability of BMIs. Method: To deal with the neural variability in online BMI control, we propose a dynamic ensemble Bayesian filter (DyEnsemble). DyEnsemble extends Bayesian filters with a dynamic measurement model, which adjusts its parameters in time adaptively with neural changes. This is achieved by learning a pool of candidate functions and dynamically weighting and assembling them according to neural signals. In this way, DyEnsemble copes with variability in signals and improves the robustness of online control. Results: Online BMI experiments with a human participant demonstrate that, compared with the velocity Kalman filter, DyEnsemble significantly improves the control accuracy (increases the success rate by 13.9% and reduces the reach time by 13.5% in the random target pursuit task) and robustness (performs more stably over different experiment days). Conclusion: Our results demonstrate the superiority of DyEnsemble in online BMI control. Significance: DyEnsemble frames a novel and flexible framework for robust neural decoding, which is beneficial to different neural decoding applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge