Ebrahim Ebrahim
multiGradICON: A Foundation Model for Multimodal Medical Image Registration
Aug 01, 2024



Abstract:Modern medical image registration approaches predict deformations using deep networks. These approaches achieve state-of-the-art (SOTA) registration accuracy and are generally fast. However, deep learning (DL) approaches are, in contrast to conventional non-deep-learning-based approaches, anatomy-specific. Recently, a universal deep registration approach, uniGradICON, has been proposed. However, uniGradICON focuses on monomodal image registration. In this work, we therefore develop multiGradICON as a first step towards universal *multimodal* medical image registration. Specifically, we show that 1) we can train a DL registration model that is suitable for monomodal *and* multimodal registration; 2) loss function randomization can increase multimodal registration accuracy; and 3) training a model with multimodal data helps multimodal generalization. Our code and the multiGradICON model are available at https://github.com/uncbiag/uniGradICON.
Harmonization Benchmarking Tool for Neuroimaging Datasets
Nov 15, 2022



Abstract:A major data pre-processing step for large, multi-site studies is to handle site effects by harmonizing data, generating a dataset that enables more powerful analyses and more robust algorithms. There is a wide variety of data harmonization techniques, but there are few tools that streamline the process of harmonizing data, comparing across techniques, and benchmarking new techniques. In this paper, we introduce HArmonization BEnchmarking Tool (HABET), an open source tool for generating harmonized images and evaluating the performance of different harmonization algorithms. To demonstrate the capabilities of HABET, we harmonize diffusion MRI images from the Adolescent Brain and Cognitive Development (ABCD) study using two different approaches, and we compare their performance.
Optimal Transport Features for Morphometric Population Analysis
Aug 11, 2022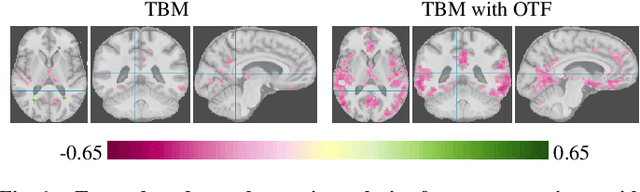
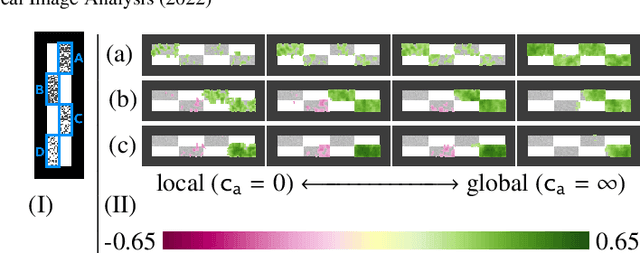
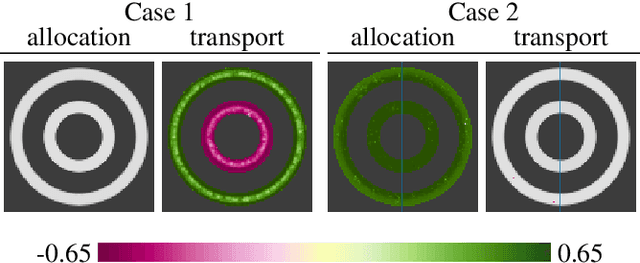
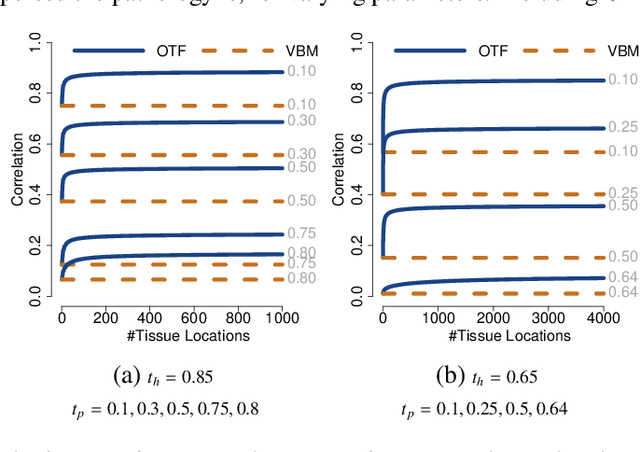
Abstract:Brain pathologies often manifest as partial or complete loss of tissue. The goal of many neuroimaging studies is to capture the location and amount of tissue changes with respect to a clinical variable of interest, such as disease progression. Morphometric analysis approaches capture local differences in the distribution of tissue or other quantities of interest in relation to a clinical variable. We propose to augment morphometric analysis with an additional feature extraction step based on unbalanced optimal transport. The optimal transport feature extraction step increases statistical power for pathologies that cause spatially dispersed tissue loss, minimizes sensitivity to shifts due to spatial misalignment or differences in brain topology, and separates changes due to volume differences from changes due to tissue location. We demonstrate the proposed optimal transport feature extraction step in the context of a volumetric morphometric analysis of the OASIS-1 study for Alzheimer's disease. The results demonstrate that the proposed approach can identify tissue changes and differences that are not otherwise measurable.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge