David S. Wishart
MassSpecGym: A benchmark for the discovery and identification of molecules
Oct 30, 2024Abstract:The discovery and identification of molecules in biological and environmental samples is crucial for advancing biomedical and chemical sciences. Tandem mass spectrometry (MS/MS) is the leading technique for high-throughput elucidation of molecular structures. However, decoding a molecular structure from its mass spectrum is exceptionally challenging, even when performed by human experts. As a result, the vast majority of acquired MS/MS spectra remain uninterpreted, thereby limiting our understanding of the underlying (bio)chemical processes. Despite decades of progress in machine learning applications for predicting molecular structures from MS/MS spectra, the development of new methods is severely hindered by the lack of standard datasets and evaluation protocols. To address this problem, we propose MassSpecGym -- the first comprehensive benchmark for the discovery and identification of molecules from MS/MS data. Our benchmark comprises the largest publicly available collection of high-quality labeled MS/MS spectra and defines three MS/MS annotation challenges: \textit{de novo} molecular structure generation, molecule retrieval, and spectrum simulation. It includes new evaluation metrics and a generalization-demanding data split, therefore standardizing the MS/MS annotation tasks and rendering the problem accessible to the broad machine learning community. MassSpecGym is publicly available at \url{https://github.com/pluskal-lab/MassSpecGym}.
Accurate, fully-automated NMR spectral profiling for metabolomics
Sep 08, 2014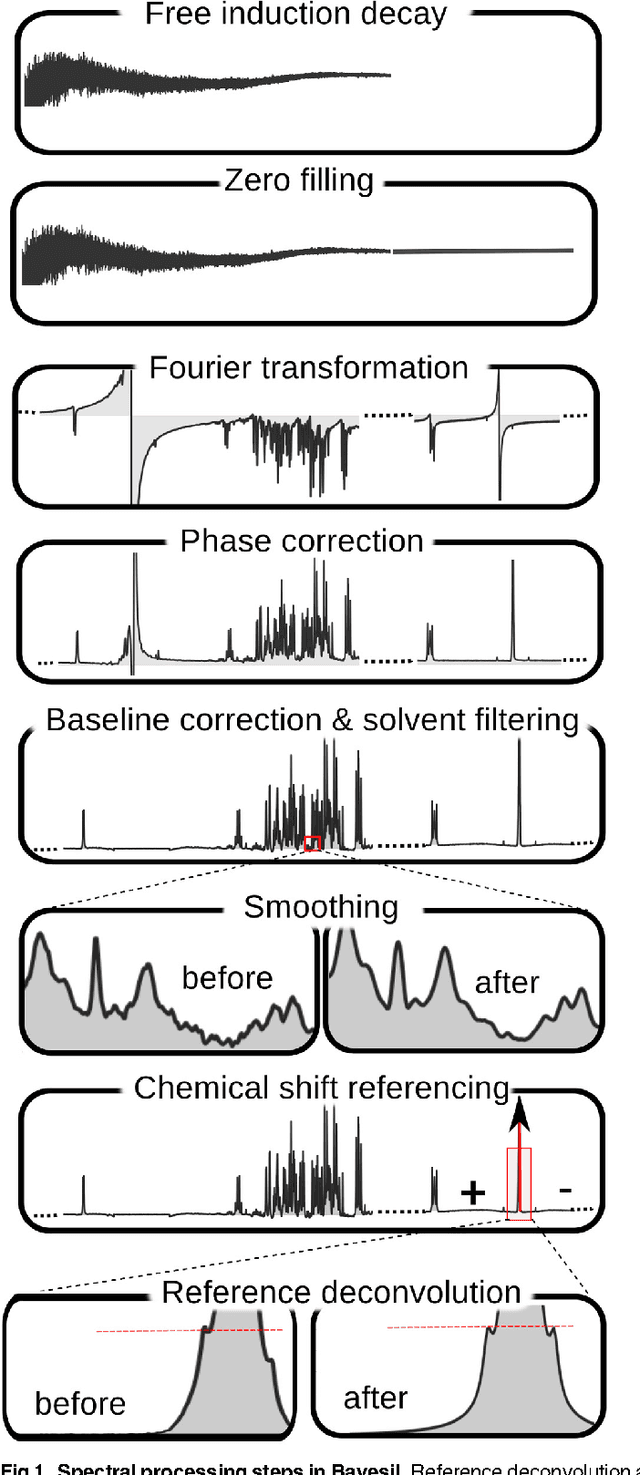
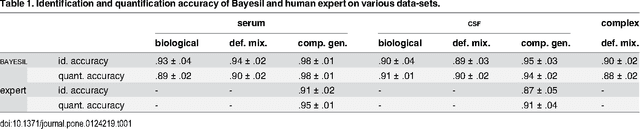
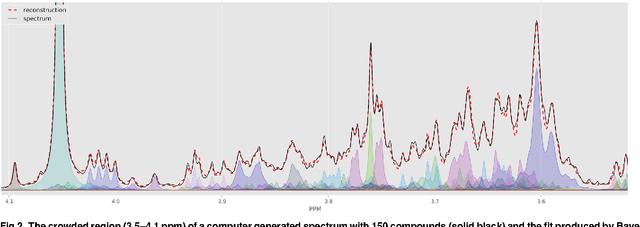
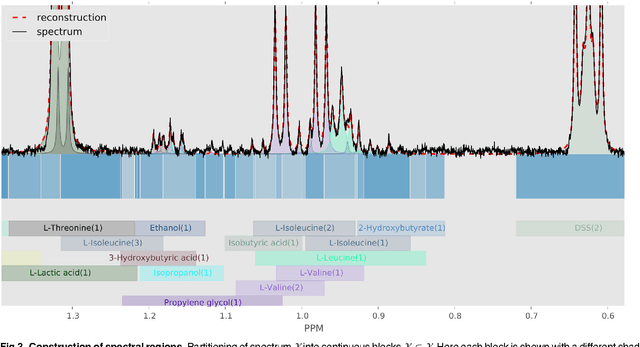
Abstract:Many diseases cause significant changes to the concentrations of small molecules (aka metabolites) that appear in a person's biofluids, which means such diseases can often be readily detected from a person's "metabolic profile". This information can be extracted from a biofluid's NMR spectrum. Today, this is often done manually by trained human experts, which means this process is relatively slow, expensive and error-prone. This paper presents a tool, Bayesil, that can quickly, accurately and autonomously produce a complex biofluid's (e.g., serum or CSF) metabolic profile from a 1D1H NMR spectrum. This requires first performing several spectral processing steps then matching the resulting spectrum against a reference compound library, which contains the "signatures" of each relevant metabolite. Many of these steps are novel algorithms and our matching step views spectral matching as an inference problem within a probabilistic graphical model that rapidly approximates the most probable metabolic profile. Our extensive studies on a diverse set of complex mixtures, show that Bayesil can autonomously find the concentration of all NMR-detectable metabolites accurately (~90% correct identification and ~10% quantification error), in <5minutes on a single CPU. These results demonstrate that Bayesil is the first fully-automatic publicly-accessible system that provides quantitative NMR spectral profiling effectively -- with an accuracy that meets or exceeds the performance of trained experts. We anticipate this tool will usher in high-throughput metabolomics and enable a wealth of new applications of NMR in clinical settings. Available at http://www.bayesil.ca.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge