Bilgin Osmanodja
Temporal Fusion Nexus: A task-agnostic multi-modal embedding model for clinical narratives and irregular time series in post-kidney transplant care
Jan 13, 2026Abstract:We introduce Temporal Fusion Nexus (TFN), a multi-modal and task-agnostic embedding model to integrate irregular time series and unstructured clinical narratives. We analysed TFN in post-kidney transplant (KTx) care, with a retrospective cohort of 3382 patients, on three key outcomes: graft loss, graft rejection, and mortality. Compared to state-of-the-art model in post KTx care, TFN achieved higher performance for graft loss (AUC 0.96 vs. 0.94) and graft rejection (AUC 0.84 vs. 0.74). In mortality prediction, TFN yielded an AUC of 0.86. TFN outperformed unimodal baselines (approx 10% AUC improvement over time series only baseline, approx 5% AUC improvement over time series with static patient data). Integrating clinical text improved performance across all tasks. Disentanglement metrics confirmed robust and interpretable latent factors in the embedding space, and SHAP-based attributions confirmed alignment with clinical reasoning. TFN has potential application in clinical tasks beyond KTx, where heterogeneous data sources, irregular longitudinal data, and rich narrative documentation are available.
One Size Fits None: Rethinking Fairness in Medical AI
Jun 17, 2025Abstract:Machine learning (ML) models are increasingly used to support clinical decision-making. However, real-world medical datasets are often noisy, incomplete, and imbalanced, leading to performance disparities across patient subgroups. These differences raise fairness concerns, particularly when they reinforce existing disadvantages for marginalized groups. In this work, we analyze several medical prediction tasks and demonstrate how model performance varies with patient characteristics. While ML models may demonstrate good overall performance, we argue that subgroup-level evaluation is essential before integrating them into clinical workflows. By conducting a performance analysis at the subgroup level, differences can be clearly identified-allowing, on the one hand, for performance disparities to be considered in clinical practice, and on the other hand, for these insights to inform the responsible development of more effective models. Thereby, our work contributes to a practical discussion around the subgroup-sensitive development and deployment of medical ML models and the interconnectedness of fairness and transparency.
When Performance is not Enough -- A Multidisciplinary View on Clinical Decision Support
Apr 27, 2022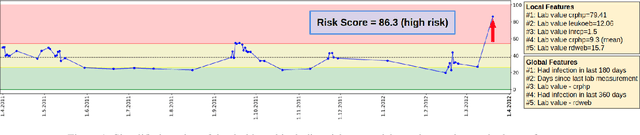
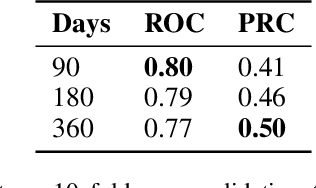
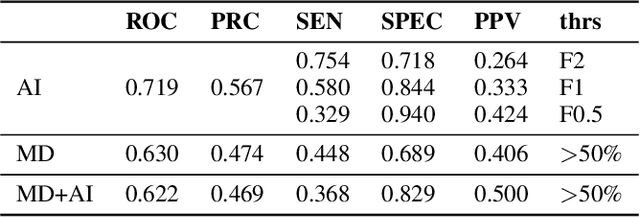
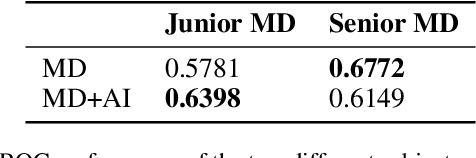
Abstract:Scientific publications about machine learning in healthcare are often about implementing novel methods and boosting the performance - at least from a computer science perspective. However, beyond such often short-lived improvements, much more needs to be taken into consideration if we want to arrive at a sustainable progress in healthcare. What does it take to actually implement such a system, make it usable for the domain expert, and possibly bring it into practical usage? Targeted at Computer Scientists, this work presents a multidisciplinary view on machine learning in medical decision support systems and covers information technology, medical, as well as ethical aspects. Along with an implemented risk prediction system in nephrology, challenges and lessons learned in a pilot project are presented.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge