Yasser Rezaeiyan
Closed-loop control of seizure activity via real-time seizure forecasting by reservoir neuromorphic computing
May 04, 2025Abstract:Closed-loop brain stimulation holds potential as personalized treatment for drug-resistant epilepsy (DRE) but still suffers from limitations that result in highly variable efficacy. First, stimulation is typically delivered upon detection of the seizure to abort rather than prevent it; second, the stimulation parameters are established by trial and error, requiring lengthy rounds of fine-tuning, which delay steady-state therapeutic efficacy. Here, we address these limitations by leveraging the potential of neuromorphic computing. We present a system capable of driving personalized free-run stimulations based on seizure forecasting, wherein each forecast triggers an electrical pulse rather than an arbitrarily predefined fixed-frequency stimulus train. We validate the system against hippocampal spheroids coupled to 3D microelectrode array as a simplified testbed, showing that it can achieve seizure reduction >97% while primarily using instantaneous stimulation frequencies within 20 Hz, well below what typically used in clinical settings. Our work demonstrates the potential of neuromorphic systems as a next-generation neuromodulation strategy for personalized DRE treatment.
NET-TEN: a silicon neuromorphic network for low-latency detection of seizures in local field potentials
Oct 19, 2022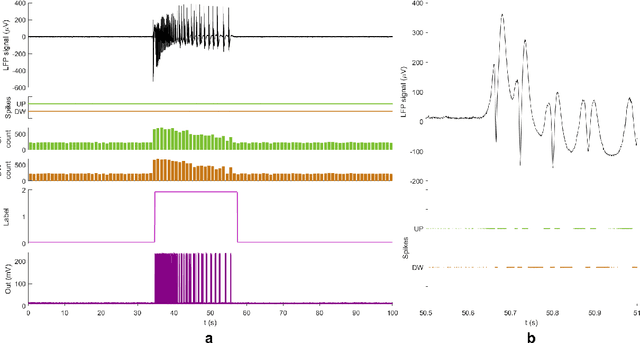
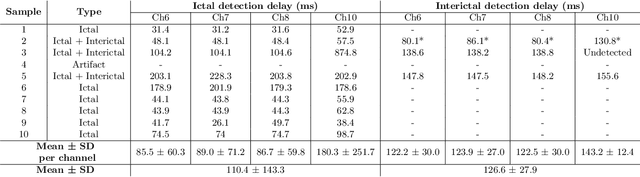
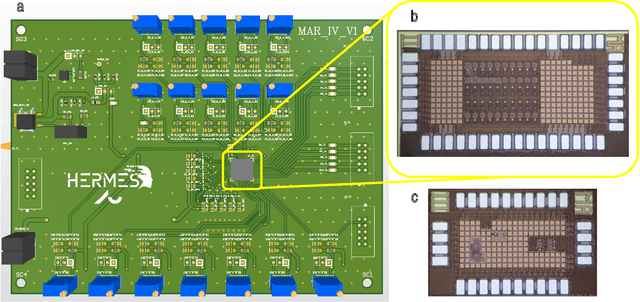
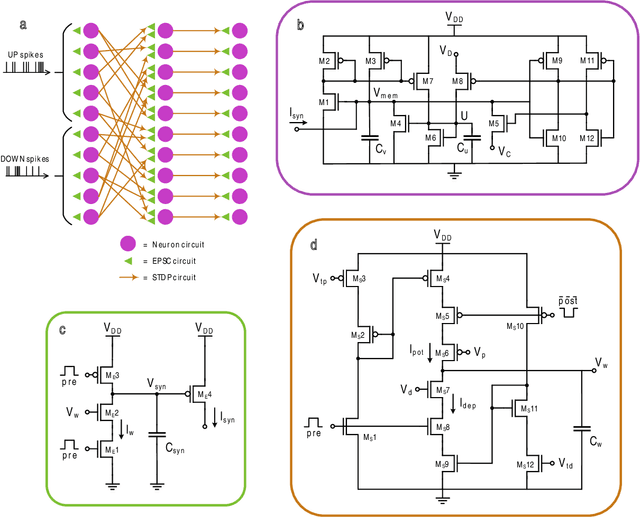
Abstract:Therapeutic intervention in neurological disorders still relies heavily on pharmacological solutions, while the treatment of patients with drug resistance remains an open challenge. This is particularly true for patients with epilepsy, 30% of whom are refractory to medications. Implantable devices for chronic recording and electrical modulation of brain activity have proved a viable alternative in such cases. To operate, the device should detect the relevant electrographic biomarkers from Local Field Potentials (LFPs) and determine the right time for stimulation. To enable timely interventions, the ideal device should attain biomarker detection with low latency while operating under low power consumption to prolong the battery life. Neuromorphic networks have progressively gained reputation as low-latency low-power computing systems, which makes them a promising candidate as processing core of next-generation implantable neural interfaces. Here we introduce a fully-analog neuromorphic device implemented in CMOS technology for analyzing LFP signals in an in vitro model of acute ictogenesis. We show that the system can detect ictal and interictal events with ms-latency and with high precision, consuming on average 3.50 nW during the task. Our work paves the way to a new generation of brain implantable devices for personalized closed-loop stimulation for epilepsy treatment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge