Philippe C. Cattin
Point Cloud Diffusion Models for Automatic Implant Generation
Mar 14, 2023Abstract:Advances in 3D printing of biocompatible materials make patient-specific implants increasingly popular. The design of these implants is, however, still a tedious and largely manual process. Existing approaches to automate implant generation are mainly based on 3D U-Net architectures on downsampled or patch-wise data, which can result in a loss of detail or contextual information. Following the recent success of Diffusion Probabilistic Models, we propose a novel approach for implant generation based on a combination of 3D point cloud diffusion models and voxelization networks. Due to the stochastic sampling process in our diffusion model, we can propose an ensemble of different implants per defect, from which the physicians can choose the most suitable one. We evaluate our method on the SkullBreak and SkullFix datasets, generating high-quality implants and achieving competitive evaluation scores.
Diffusion Models for Contrast Harmonization of Magnetic Resonance Images
Mar 14, 2023



Abstract:Magnetic resonance (MR) images from multiple sources often show differences in image contrast related to acquisition settings or the used scanner type. For long-term studies, longitudinal comparability is essential but can be impaired by these contrast differences, leading to biased results when using automated evaluation tools. This study presents a diffusion model-based approach for contrast harmonization. We use a data set consisting of scans of 18 Multiple Sclerosis patients and 22 healthy controls. Each subject was scanned in two MR scanners of different magnetic field strengths (1.5 T and 3 T), resulting in a paired data set that shows scanner-inherent differences. We map images from the source contrast to the target contrast for both directions, from 3 T to 1.5 T and from 1.5 T to 3 T. As we only want to change the contrast, not the anatomical information, our method uses the original image to guide the image-to-image translation process by adding structural information. The aim is that the mapped scans display increased comparability with scans of the target contrast for downstream tasks. We evaluate this method for the task of segmentation of cerebrospinal fluid, grey matter and white matter. Our method achieves good and consistent results for both directions of the mapping.
Improved distinct bone segmentation in upper-body CT through multi-resolution networks
Jan 31, 2023Abstract:Purpose: Automated distinct bone segmentation from CT scans is widely used in planning and navigation workflows. U-Net variants are known to provide excellent results in supervised semantic segmentation. However, in distinct bone segmentation from upper body CTs a large field of view and a computationally taxing 3D architecture are required. This leads to low-resolution results lacking detail or localisation errors due to missing spatial context when using high-resolution inputs. Methods: We propose to solve this problem by using end-to-end trainable segmentation networks that combine several 3D U-Nets working at different resolutions. Our approach, which extends and generalizes HookNet and MRN, captures spatial information at a lower resolution and skips the encoded information to the target network, which operates on smaller high-resolution inputs. We evaluated our proposed architecture against single resolution networks and performed an ablation study on information concatenation and the number of context networks. Results: Our proposed best network achieves a median DSC of 0.86 taken over all 125 segmented bone classes and reduces the confusion among similar-looking bones in different locations. These results outperform our previously published 3D U-Net baseline results on the task and distinct-bone segmentation results reported by other groups. Conclusion: The presented multi-resolution 3D U-Nets address current shortcomings in bone segmentation from upper-body CT scans by allowing for capturing a larger field of view while avoiding the cubic growth of the input pixels and intermediate computations that quickly outgrow the computational capacities in 3D. The approach thus improves the accuracy and efficiency of distinct bone segmentation from upper-body CT.
Position Regression for Unsupervised Anomaly Detection
Jan 19, 2023Abstract:In recent years, anomaly detection has become an essential field in medical image analysis. Most current anomaly detection methods for medical images are based on image reconstruction. In this work, we propose a novel anomaly detection approach based on coordinate regression. Our method estimates the position of patches within a volume, and is trained only on data of healthy subjects. During inference, we can detect and localize anomalies by considering the error of the position estimate of a given patch. We apply our method to 3D CT volumes and evaluate it on patients with intracranial haemorrhages and cranial fractures. The results show that our method performs well in detecting these anomalies. Furthermore, we show that our method requires less memory than comparable approaches that involve image reconstruction. This is highly relevant for processing large 3D volumes, for instance, CT or MRI scans.
Using Supervised Deep-Learning to Model Edge-FBG Shape Sensors
Oct 28, 2022Abstract:Continuum robots in robot-assisted minimally invasive surgeries provide adequate access to target anatomies that are not directly reachable through small incisions. Achieving precise and reliable motion control of such snake-like manipulators necessitates an accurate navigation system that requires no line-of-sight and is immune to electromagnetic noises. Fiber Bragg Grating (FBG) shape sensors, particularly edge-FBGs, are promising tools for this task. However, in edge-FBG sensors, the intensity ratio between Bragg wavelengths carries the strain information that can be affected by undesired bending-related phenomena, making standard characterization techniques less suitable for these sensors. We showed in our previous work that a deep learning model has the potential to extract the strain information from the full edge-FBG spectrum and accurately predict the sensor's shape. In this paper, we conduct a more thorough investigation to find a suitable architectural design with lower prediction errors. We use the Hyperband algorithm to search for optimal hyperparameters in two steps. First, we limit the search space to layer settings, where the best-performing configuration gets selected. Then, we modify the search space for tuning the training and loss calculation hyperparameters. We also analyze various data transformations on the input and output variables, as data rescaling can directly influence the model's performance. Moreover, we performed discriminative training using Siamese network architecture that employs two CNNs with identical parameters to learn similarity metrics between the spectra of similar target values. The best-performing network architecture among all evaluated configurations can predict the sensor's shape with a median tip error of 3.11 mm.
The secret role of undesired physical effects in accurate shape sensing with eccentric FBGs
Oct 28, 2022Abstract:Fiber optic shape sensors have enabled unique advances in various navigation tasks, from medical tool tracking to industrial applications. Eccentric fiber Bragg gratings (FBG) are cheap and easy-to-fabricate shape sensors that are often interrogated with simple setups. However, using low-cost interrogation systems for such intensity-based quasi-distributed sensors introduces further complications to the sensor's signal. Therefore, eccentric FBGs have not been able to accurately estimate complex multi-bend shapes. Here, we present a novel technique to overcome these limitations and provide accurate and precise shape estimation in eccentric FBG sensors. We investigate the most important bending-induced effects in curved optical fibers that are usually eliminated in intensity-based fiber sensors. These effects contain shape deformation information with a higher spatial resolution that we are now able to extract using deep learning techniques. We design a deep learning model based on a convolutional neural network that is trained to predict shapes given the sensor's spectra. We also provide a visual explanation, highlighting wavelength elements whose intensities are more relevant in making shape predictions. These findings imply that deep learning techniques benefit from the bending-induced effects that impact the desired signal in a complex manner. This is the first step toward cheap yet accurate fiber shape sensing solutions.
The Swiss Army Knife for Image-to-Image Translation: Multi-Task Diffusion Models
Apr 06, 2022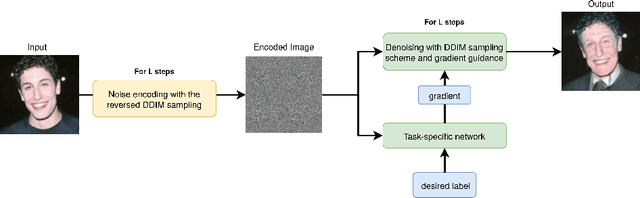



Abstract:Recently, diffusion models were applied to a wide range of image analysis tasks. We build on a method for image-to-image translation using denoising diffusion implicit models and include a regression problem and a segmentation problem for guiding the image generation to the desired output. The main advantage of our approach is that the guidance during the denoising process is done by an external gradient. Consequently, the diffusion model does not need to be retrained for the different tasks on the same dataset. We apply our method to simulate the aging process on facial photos using a regression task, as well as on a brain magnetic resonance (MR) imaging dataset for the simulation of brain tumor growth. Furthermore, we use a segmentation model to inpaint tumors at the desired location in healthy slices of brain MR images. We achieve convincing results for all problems.
Diffusion Models for Medical Anomaly Detection
Mar 08, 2022
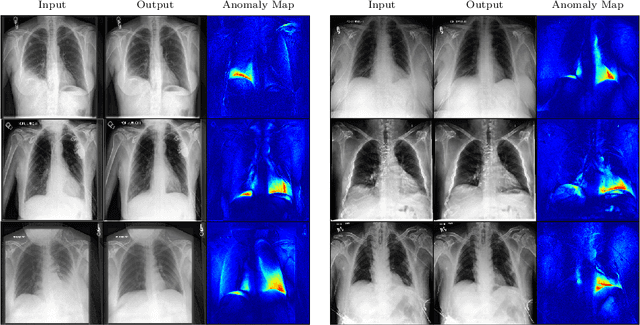
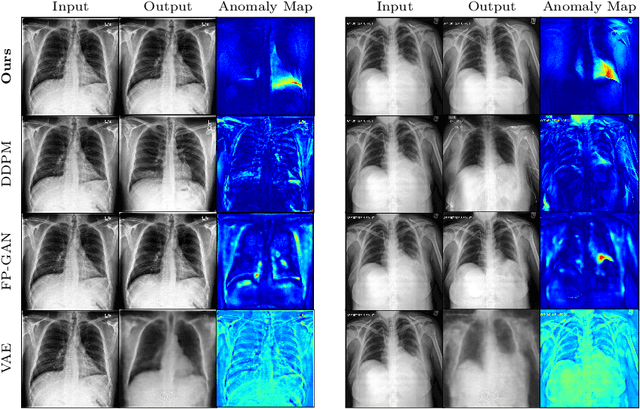
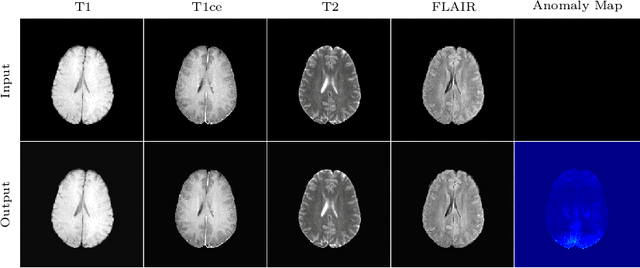
Abstract:In medical applications, weakly supervised anomaly detection methods are of great interest, as only image-level annotations are required for training. Current anomaly detection methods mainly rely on generative adversarial networks or autoencoder models. Those models are often complicated to train or have difficulties to preserve fine details in the image. We present a novel weakly supervised anomaly detection method based on denoising diffusion implicit models. We combine the deterministic iterative noising and denoising scheme with classifier guidance for image-to-image translation between diseased and healthy subjects. Our method generates very detailed anomaly maps without the need for a complex training procedure. We evaluate our method on the BRATS2020 dataset for brain tumor detection and the CheXpert dataset for detecting pleural effusions.
Diffusion Models for Implicit Image Segmentation Ensembles
Dec 27, 2021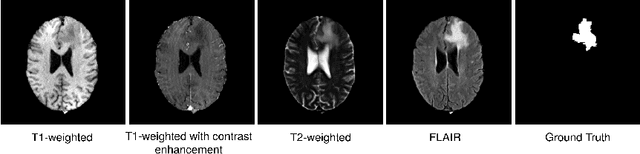
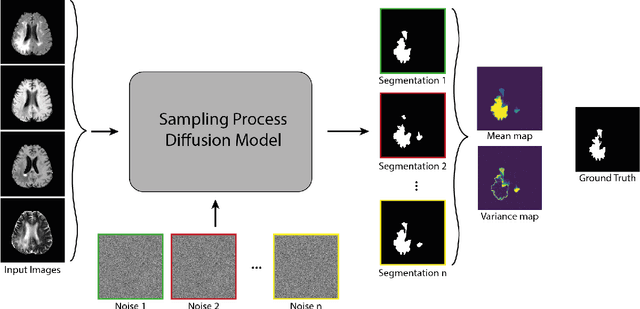

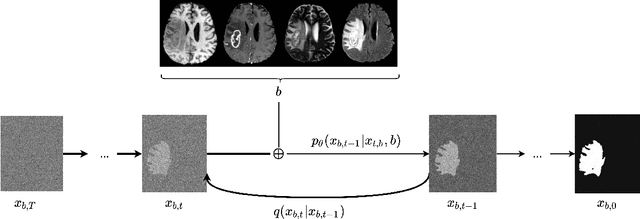
Abstract:Diffusion models have shown impressive performance for generative modelling of images. In this paper, we present a novel semantic segmentation method based on diffusion models. By modifying the training and sampling scheme, we show that diffusion models can perform lesion segmentation of medical images. To generate an image specific segmentation, we train the model on the ground truth segmentation, and use the image as a prior during training and in every step during the sampling process. With the given stochastic sampling process, we can generate a distribution of segmentation masks. This property allows us to compute pixel-wise uncertainty maps of the segmentation, and allows an implicit ensemble of segmentations that increases the segmentation performance. We evaluate our method on the BRATS2020 dataset for brain tumor segmentation. Compared to state-of-the-art segmentation models, our approach yields good segmentation results and, additionally, detailed uncertainty maps.
Learn to Ignore: Domain Adaptation for Multi-Site MRI Analysis
Oct 13, 2021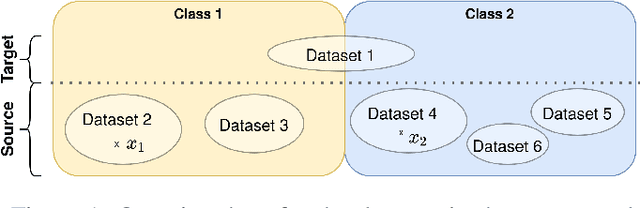
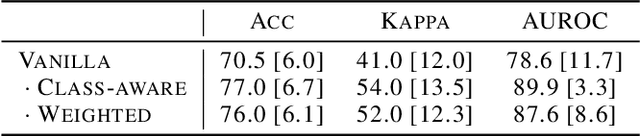
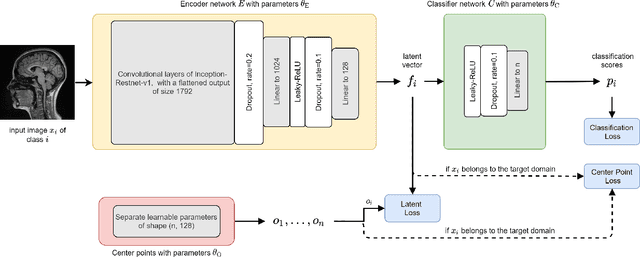
Abstract:Limited availability of large image datasets is a major issue in the development of accurate and generalizable machine learning methods in medicine. The limitations in the amount of data are mainly due to the use of different acquisition protocols, different hardware, and data privacy. At the same time, training a classification model on a small dataset leads to a poor generalization quality of the model. To overcome this issue, a combination of various image datasets of different provenance is often used, e.g., multi-site studies. However, if an additional dataset does not include all classes of the task, the learning of the classification model can be biased to the device or place of acquisition. This is especially the case for Magnetic Resonance (MR) images, where different MR scanners introduce a bias that limits the performance of the model. In this paper, we present a novel method that learns to ignore the scanner-related features present in the images, while learning features relevant for the classification task. We focus on a real-world scenario, where only a small dataset provides images of all classes. We exploit this circumstance by introducing specific additional constraints on the latent space, which lead the focus on disease-related rather than scanner-specific features. Our method Learn to Ignore outperforms state-of-the-art domain adaptation methods on a multi-site MRI dataset on a classification task between Multiple Sclerosis patients and healthy subjects.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge