Parvez Ahmad
SAMA-UNet: Enhancing Medical Image Segmentation with Self-Adaptive Mamba-Like Attention and Causal-Resonance Learning
May 21, 2025Abstract:Medical image segmentation plays an important role in various clinical applications, but existing models often struggle with the computational inefficiencies and challenges posed by complex medical data. State Space Sequence Models (SSMs) have demonstrated promise in modeling long-range dependencies with linear computational complexity, yet their application in medical image segmentation remains hindered by incompatibilities with image tokens and autoregressive assumptions. Moreover, it is difficult to achieve a balance in capturing both local fine-grained information and global semantic dependencies. To address these challenges, we introduce SAMA-UNet, a novel architecture for medical image segmentation. A key innovation is the Self-Adaptive Mamba-like Aggregated Attention (SAMA) block, which integrates contextual self-attention with dynamic weight modulation to prioritise the most relevant features based on local and global contexts. This approach reduces computational complexity and improves the representation of complex image features across multiple scales. We also suggest the Causal-Resonance Multi-Scale Module (CR-MSM), which enhances the flow of information between the encoder and decoder by using causal resonance learning. This mechanism allows the model to automatically adjust feature resolution and causal dependencies across scales, leading to better semantic alignment between the low-level and high-level features in U-shaped architectures. Experiments on MRI, CT, and endoscopy images show that SAMA-UNet performs better in segmentation accuracy than current methods using CNN, Transformer, and Mamba. The implementation is publicly available at GitHub.
HI-Net: Hyperdense Inception 3D UNet for Brain Tumor Segmentation
Dec 12, 2020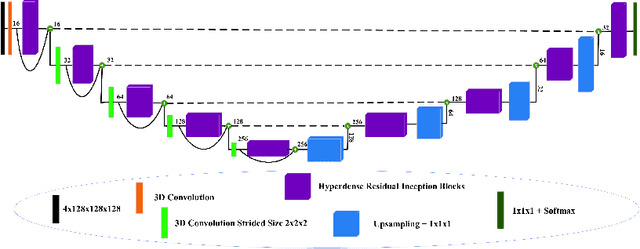
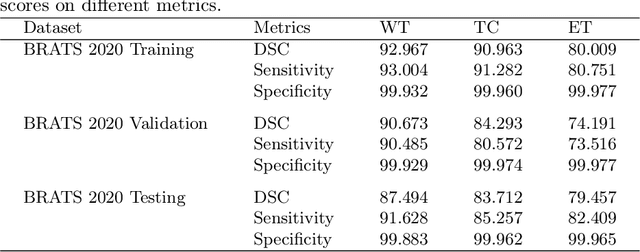
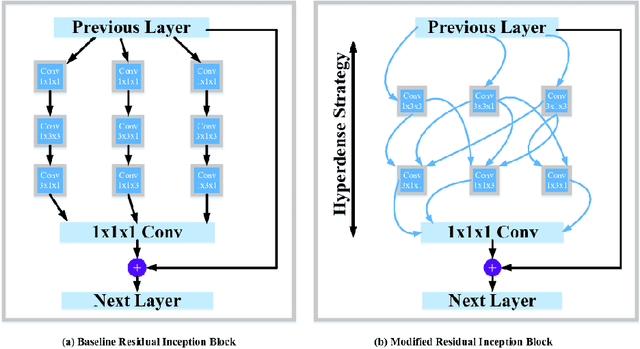
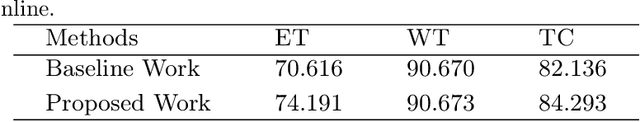
Abstract:The brain tumor segmentation task aims to classify tissue into the whole tumor (WT), tumor core (TC), and enhancing tumor (ET) classes using multimodel MRI images. Quantitative analysis of brain tumors is critical for clinical decision making. While manual segmentation is tedious, time-consuming, and subjective, this task is at the same time very challenging to automatic segmentation methods. Thanks to the powerful learning ability, convolutional neural networks (CNNs), mainly fully convolutional networks, have shown promising brain tumor segmentation. This paper further boosts the performance of brain tumor segmentation by proposing hyperdense inception 3D UNet (HI-Net), which captures multi-scale information by stacking factorization of 3D weighted convolutional layers in the residual inception block. We use hyper dense connections among factorized convolutional layers to extract more contexual information, with the help of features reusability. We use a dice loss function to cope with class imbalances. We validate the proposed architecture on the multi-modal brain tumor segmentation challenges (BRATS) 2020 testing dataset. Preliminary results on the BRATS 2020 testing set show that achieved by our proposed approach, the dice (DSC) scores of ET, WT, and TC are 0.79457, 0.87494, and 0.83712, respectively.
Context Aware 3D UNet for Brain Tumor Segmentation
Oct 25, 2020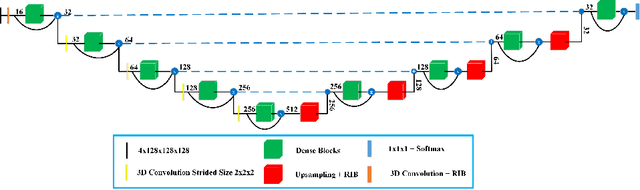
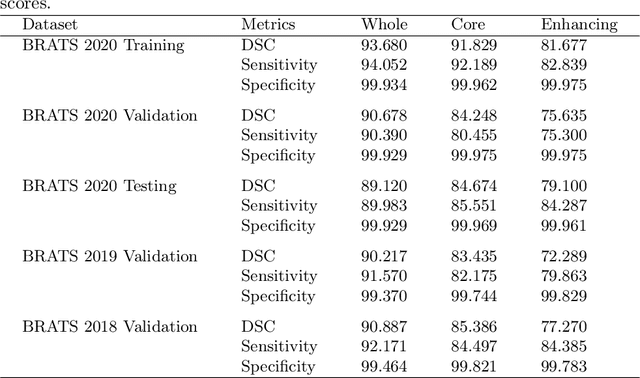
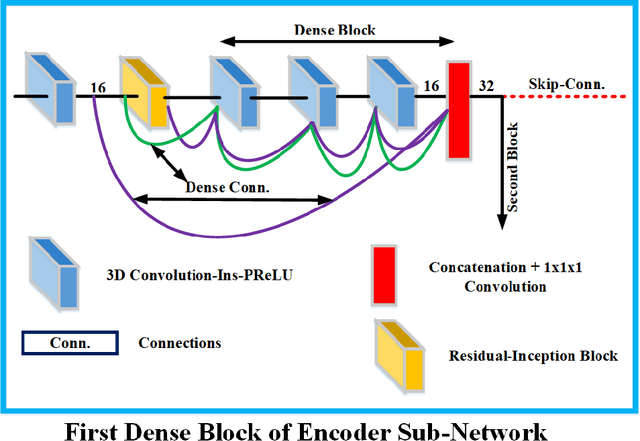
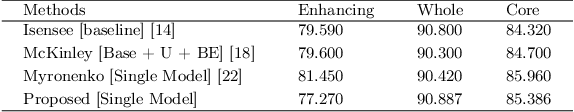
Abstract:Deep convolutional neural network (CNN) achieves remarkable performance for medical image analysis. UNet is the primary source in the performance of 3D CNN architectures for medical imaging tasks, including brain tumor segmentation. The skip connection in the UNet architecture concatenates features from both encoder and decoder paths to extract multi-contexual information from image data. The multi-scaled features play an essential role in brain tumor segmentation. However, the limited use of features can degrade the performance of the UNet approach for segmentation. In this paper, we propose a modified UNet architecture for brain tumor segmentation. In the proposed architecture, we used densely connected blocks in both encoder and decoder paths to extract multi-contexual information from the concept of feature reusability. The proposed residual inception blocks (RIB) are used to extract local and global information by merging features of different kernel sizes. We validate the proposed architecture on the multimodal brain tumor segmentation challenges (BRATS) 2020 testing dataset. The dice (DSC) scores of the whole tumor (WT), tumor core (TC), and enhancement tumor (ET) are 89.12%, 84.74%, and 79.12%, respectively. Our proposed work is in the top ten methods based on the dice scores of the testing dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge