Keiichi Nakagawa
Iterative CT Reconstruction via Latent Variable Optimization of Shallow Diffusion Models
Aug 06, 2024Abstract:Image generative AI has garnered significant attention in recent years. In particular, the diffusion model, a core component of recent generative AI, produces high-quality images with rich diversity. In this study, we propose a novel CT reconstruction method by combining the denoising diffusion probabilistic model with iterative CT reconstruction. In sharp contrast to previous studies, we optimize the fidelity loss of CT reconstruction with respect to the latent variable of the diffusion model, instead of the image and model parameters. To suppress anatomical structure changes produced by the diffusion model, we shallow the diffusion and reverse processes, and fix a set of added noises in the reverse process to make it deterministic during inference. We demonstrate the effectiveness of the proposed method through sparse view CT reconstruction of 1/10 view projection data. Despite the simplicity of the implementation, the proposed method shows the capability of reconstructing high-quality images while preserving the patient's anatomical structure, and outperforms existing methods including iterative reconstruction, iterative reconstruction with total variation, and the diffusion model alone in terms of quantitative indices such as SSIM and PSNR. We also explore further sparse view CT using 1/20 view projection data with the same trained diffusion model. As the number of iterations increases, image quality improvement comparable to that of 1/10 sparse view CT reconstruction is achieved. In principle, the proposed method can be widely applied not only to CT but also to other imaging modalities such as MRI, PET, and SPECT.
Training deep cross-modality conversion models with a small amount of data and its application to MVCT to kVCT conversion
Jul 12, 2021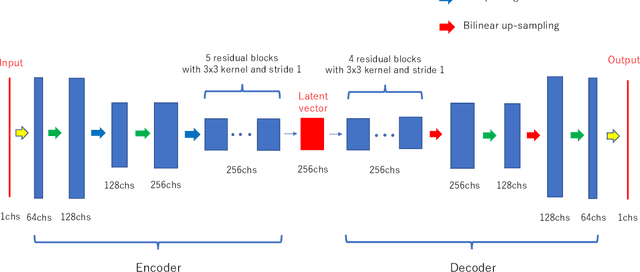
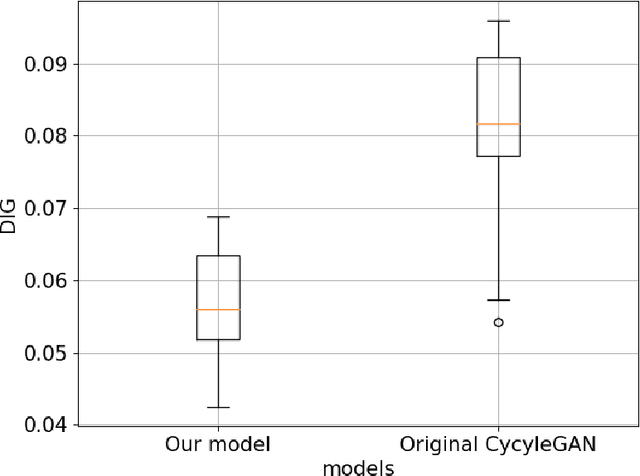
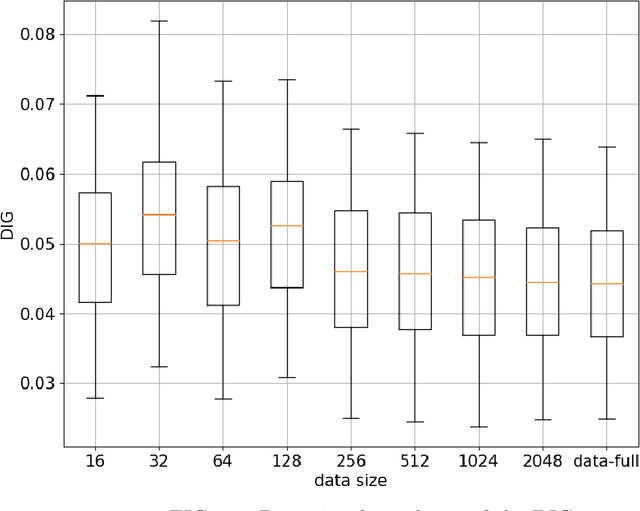
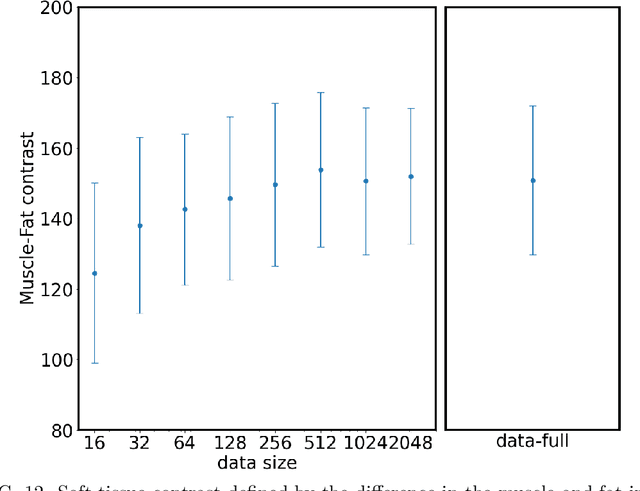
Abstract:Deep-learning-based image processing has emerged as a valuable tool in recent years owing to its high performance. However, the quality of deep-learning-based methods relies heavily on the amount of training data, and the cost of acquiring a large amount of data is often prohibitive in medical fields. Therefore, we performed CT modality conversion based on deep learning requiring only a small number of unsupervised images. The proposed method is based on generative adversarial networks (GANs) with several extensions tailored for CT images. This method emphasizes the preservation of the structure in the processed images and reduction in the amount of training data. This method was applied to realize the conversion of mega-voltage computed tomography (MVCT) to kilo-voltage computed tomography (kVCT) images. Training was performed using several datasets acquired from patients with head and neck cancer. The size of the datasets ranged from 16 slices (for two patients) to 2745 slices (for 137 patients) of MVCT and 2824 slices of kVCT for 98 patients. The quality of the processed MVCT images was considerably enhanced, and the structural changes in the images were minimized. With an increase in the size of training data, the image quality exhibited a satisfactory convergence from a few hundred slices. In addition to statistical and visual evaluations, these results were clinically evaluated by medical doctors in terms of the accuracy of contouring. We developed an MVCT to kVCT conversion model based on deep learning, which can be trained using a few hundred unpaired images. The stability of the model against the change in the data size was demonstrated. This research promotes the reliable use of deep learning in clinical medicine by partially answering the commonly asked questions: "Is our data enough? How much data must we prepare?"
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge