Hariprasad Korsapati
Reinforcement Learning for Out-of-Distribution Reasoning in LLMs: An Empirical Study on Diagnosis-Related Group Coding
May 28, 2025Abstract:Diagnosis-Related Group (DRG) codes are essential for hospital reimbursement and operations but require labor-intensive assignment. Large Language Models (LLMs) struggle with DRG coding due to the out-of-distribution (OOD) nature of the task: pretraining corpora rarely contain private clinical or billing data. We introduce DRG-Sapphire, which uses large-scale reinforcement learning (RL) for automated DRG coding from clinical notes. Built on Qwen2.5-7B and trained with Group Relative Policy Optimization (GRPO) using rule-based rewards, DRG-Sapphire introduces a series of RL enhancements to address domain-specific challenges not seen in previous mathematical tasks. Our model achieves state-of-the-art accuracy on the MIMIC-IV benchmark and generates physician-validated reasoning for DRG assignments, significantly enhancing explainability. Our study further sheds light on broader challenges of applying RL to knowledge-intensive, OOD tasks. We observe that RL performance scales approximately linearly with the logarithm of the number of supervised fine-tuning (SFT) examples, suggesting that RL effectiveness is fundamentally constrained by the domain knowledge encoded in the base model. For OOD tasks like DRG coding, strong RL performance requires sufficient knowledge infusion prior to RL. Consequently, scaling SFT may be more effective and computationally efficient than scaling RL alone for such tasks.
Process-Supervised Reward Models for Clinical Note Generation: A Scalable Approach Guided by Domain Expertise
Dec 17, 2024



Abstract:Process-supervised reward models (PRMs), which verify large language model (LLM) outputs step-by-step, have achieved significant success in mathematical and coding problems. However, their application to other domains remains largely unexplored. In this work, we train a PRM to provide step-level reward signals for clinical notes generated by LLMs from patient-doctor dialogues. Guided by real-world clinician expertise, we carefully designed step definitions for clinical notes and utilized Gemini-Pro 1.5 to automatically generate process supervision data at scale. Our proposed PRM, trained on the LLaMA-3.1 8B instruct model, demonstrated superior performance compared to Gemini-Pro 1.5 and an outcome-supervised reward model (ORM) across two key evaluations: (1) the accuracy of selecting gold-reference samples from error-containing samples, achieving 98.8% (versus 61.3% for ORM and 93.8% for Gemini-Pro 1.5), and (2) the accuracy of selecting physician-preferred notes, achieving 56.2% (compared to 51.2% for ORM and 50.0% for Gemini-Pro 1.5). Additionally, we conducted ablation studies to determine optimal loss functions and data selection strategies, along with physician reader studies to explore predictors of downstream Best-of-N performance. Our promising results suggest the potential of PRMs to extend beyond the clinical domain, offering a scalable and effective solution for diverse generative tasks.
Towards Adapting Open-Source Large Language Models for Expert-Level Clinical Note Generation
Apr 25, 2024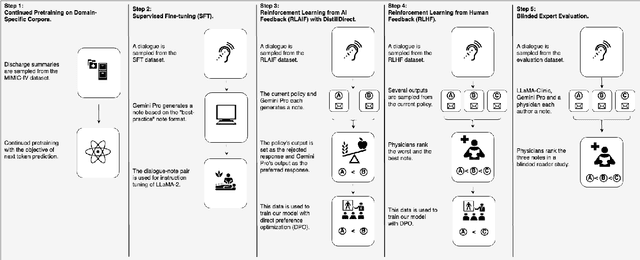
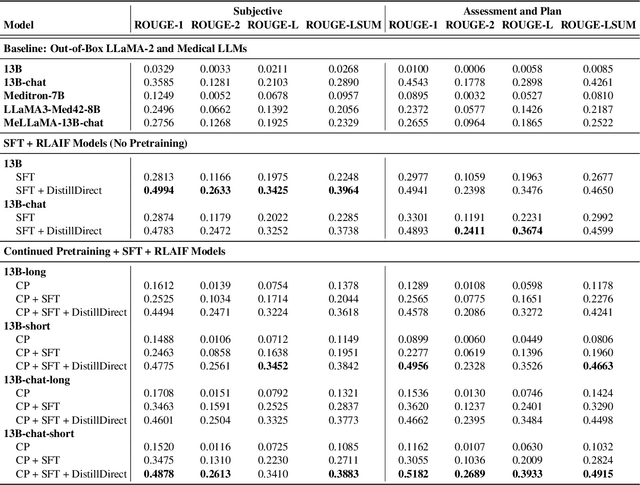
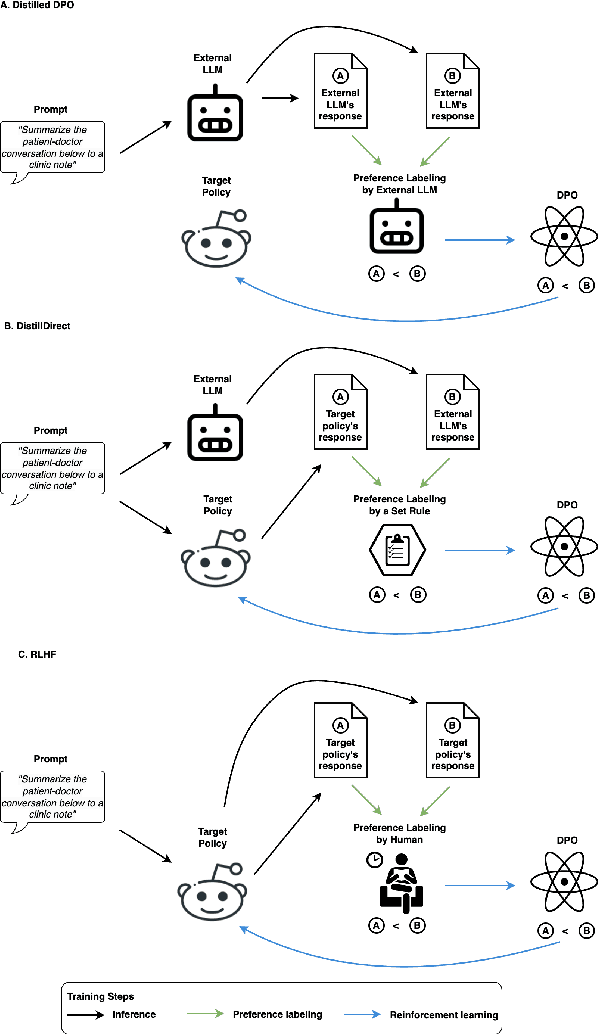
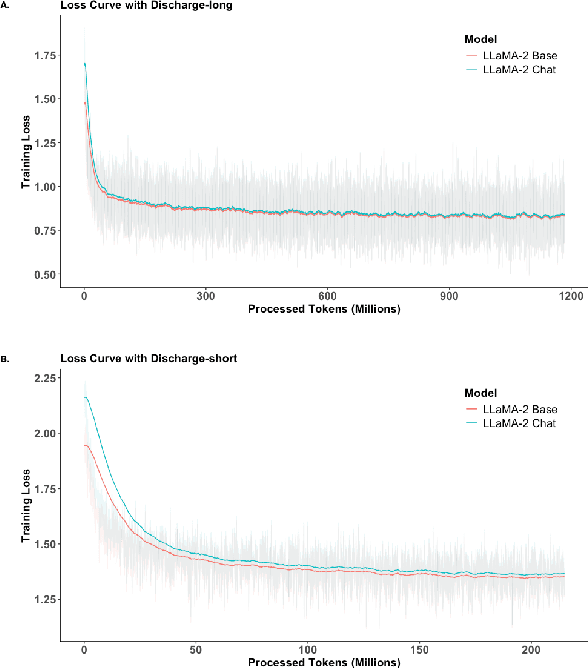
Abstract:Large Language Models (LLMs) have shown promising capabilities in handling clinical text summarization tasks. In this study, we demonstrate that a small open-source LLM can be effectively trained to generate high-quality clinical notes from outpatient patient-doctor dialogues. We achieve this through a comprehensive domain- and task-specific adaptation process for the LLaMA-2 13 billion parameter model. This process incorporates continued pre-training, supervised fine-tuning, and reinforcement learning from both AI and human feedback. We introduced an enhanced approach, termed DistillDirect, for performing on-policy reinforcement learning with Gemini Pro serving as the teacher model. Our resulting model, LLaMA-Clinic, is capable of generating clinical notes that are comparable in quality to those authored by physicians. In a blinded physician reader study, the majority (90.4%) of individual evaluations rated the notes generated by LLaMA-Clinic as "acceptable" or higher across all three criteria: real-world readiness, completeness, and accuracy. Notably, in the more challenging "Assessment and Plan" section, LLaMA-Clinic scored higher (4.2/5) in real-world readiness compared to physician-authored notes (4.1/5). Additionally, we identified caveats in public clinical note datasets, such as ACI-BENCH. We highlight key considerations for future clinical note-generation tasks, emphasizing the importance of pre-defining a best-practice note format. Overall, our research demonstrates the potential and feasibility of training smaller, open-source LLMs to assist with clinical documentation, capitalizing on healthcare institutions' access to patient records and domain expertise. We have made our newly created synthetic clinic dialogue-note dataset and the physician feedback dataset publicly available to foster future research in this field.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge