Debbie Zhao
A Computational Pipeline for Advanced Analysis of 4D Flow MRI in the Left Atrium
May 14, 2025Abstract:The left atrium (LA) plays a pivotal role in modulating left ventricular filling, but our comprehension of its hemodynamics is significantly limited by the constraints of conventional ultrasound analysis. 4D flow magnetic resonance imaging (4D Flow MRI) holds promise for enhancing our understanding of atrial hemodynamics. However, the low velocities within the LA and the limited spatial resolution of 4D Flow MRI make analyzing this chamber challenging. Furthermore, the absence of dedicated computational frameworks, combined with diverse acquisition protocols and vendors, complicates gathering large cohorts for studying the prognostic value of hemodynamic parameters provided by 4D Flow MRI. In this study, we introduce the first open-source computational framework tailored for the analysis of 4D Flow MRI in the LA, enabling comprehensive qualitative and quantitative analysis of advanced hemodynamic parameters. Our framework proves robust to data from different centers of varying quality, producing high-accuracy automated segmentations (Dice $>$ 0.9 and Hausdorff 95 $<$ 3 mm), even with limited training data. Additionally, we conducted the first comprehensive assessment of energy, vorticity, and pressure parameters in the LA across a spectrum of disorders to investigate their potential as prognostic biomarkers.
4DFlowNet: Super-Resolution 4D Flow MRI using Deep Learning and Computational Fluid Dynamics
Apr 15, 2020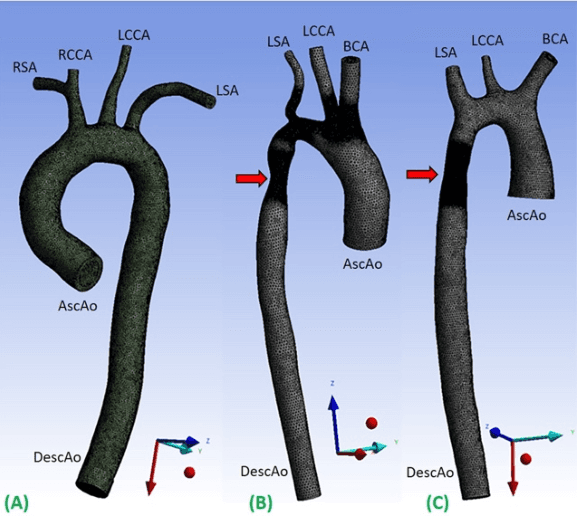
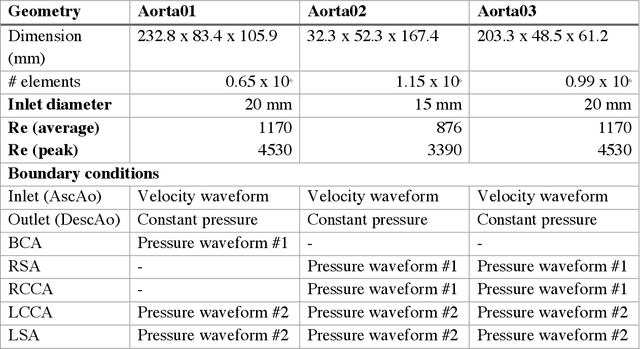
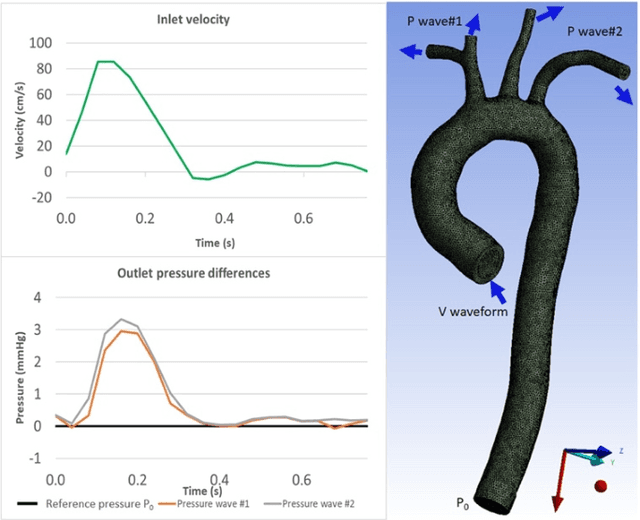
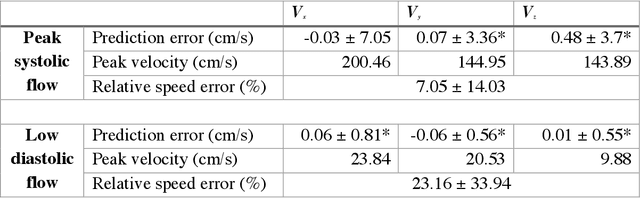
Abstract:4D-flow magnetic resonance imaging (MRI) is an emerging imaging technique where spatiotemporal 3D blood velocity can be captured with full volumetric coverage in a single non-invasive examination. This enables qualitative and quantitative analysis of hemodynamic flow parameters of the heart and great vessels. An increase in the image resolution would provide more accuracy and allow better assessment of the blood flow, especially for patients with abnormal flows. However, this must be balanced with increasing imaging time. The recent success of deep learning in generating super resolution images shows promise for implementation in medical images. We utilized computational fluid dynamics simulations to generate fluid flow simulations and represent them as synthetic 4D flow MRI data. We built our training dataset to mimic actual 4D flow MRI data with its corresponding noise distribution. Our novel 4DFlowNet network was trained on this synthetic 4D flow data and was capable in producing noise-free super resolution 4D flow phase images with upsample factor of 2. We also tested the 4DFlowNet in actual 4D flow MR images of a phantom and normal volunteer data, and demonstrated comparable results with the actual flow rate measurements giving an absolute relative error of 0.6 to 5.8% and 1.1 to 3.8% in the phantom data and normal volunteer data, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge