Danilo Babin
Clinical Validation of Deep Learning for Real-Time Tissue Oxygenation Estimation Using Spectral Imaging
May 23, 2025Abstract:Accurate, real-time monitoring of tissue ischemia is crucial to understand tissue health and guide surgery. Spectral imaging shows great potential for contactless and intraoperative monitoring of tissue oxygenation. Due to the difficulty of obtaining direct reference oxygenation values, conventional methods are based on linear unmixing techniques. These are prone to assumptions and these linear relations may not always hold in practice. In this work, we present deep learning approaches for real-time tissue oxygenation estimation using Monte-Carlo simulated spectra. We train a fully connected neural network (FCN) and a convolutional neural network (CNN) for this task and propose a domain-adversarial training approach to bridge the gap between simulated and real clinical spectral data. Results demonstrate that these deep learning models achieve a higher correlation with capillary lactate measurements, a well-known marker of hypoxia, obtained during spectral imaging in surgery, compared to traditional linear unmixing. Notably, domain-adversarial training effectively reduces the domain gap, optimizing performance in real clinical settings.
Using the Polar Transform for Efficient Deep Learning-Based Aorta Segmentation in CTA Images
Jun 21, 2022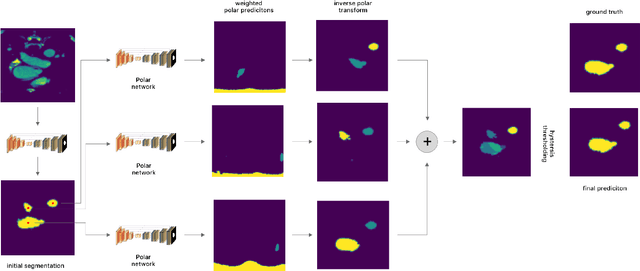
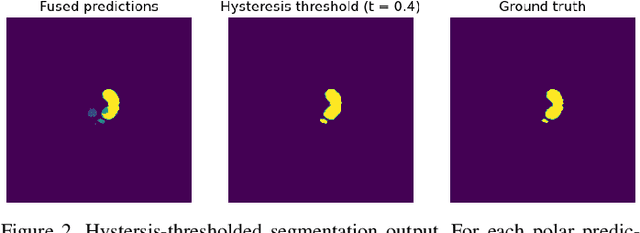
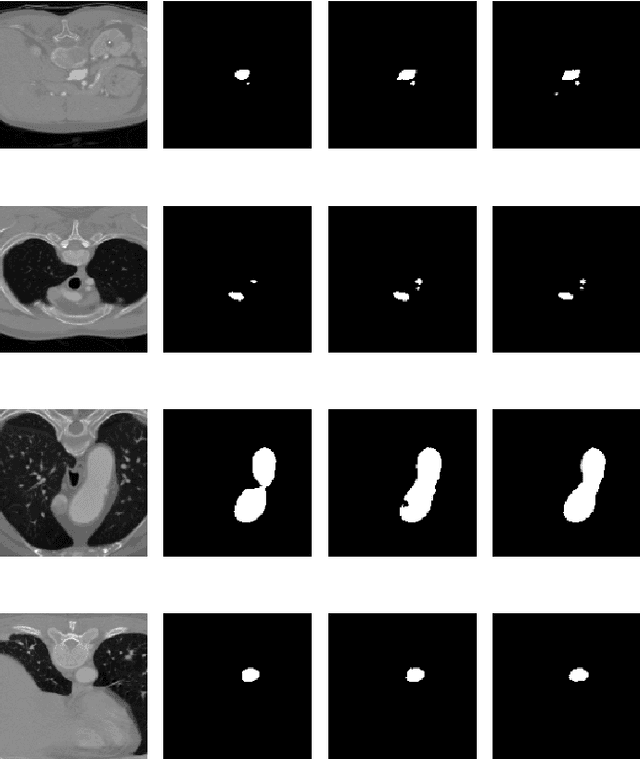
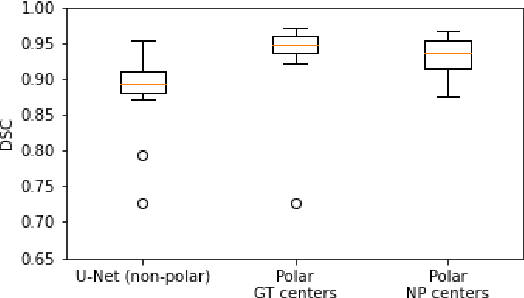
Abstract:Medical image segmentation often requires segmenting multiple elliptical objects on a single image. This includes, among other tasks, segmenting vessels such as the aorta in axial CTA slices. In this paper, we present a general approach to improving the semantic segmentation performance of neural networks in these tasks and validate our approach on the task of aorta segmentation. We use a cascade of two neural networks, where one performs a rough segmentation based on the U-Net architecture and the other performs the final segmentation on polar image transformations of the input. Connected component analysis of the rough segmentation is used to construct the polar transformations, and predictions on multiple transformations of the same image are fused using hysteresis thresholding. We show that this method improves aorta segmentation performance without requiring complex neural network architectures. In addition, we show that our approach improves robustness and pixel-level recall while achieving segmentation performance in line with the state of the art.
A Survey of Left Atrial Appendage Segmentation and Analysis in 3D and 4D Medical Images
May 13, 2022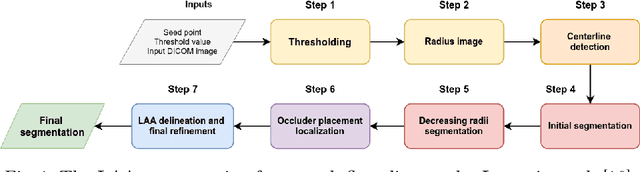
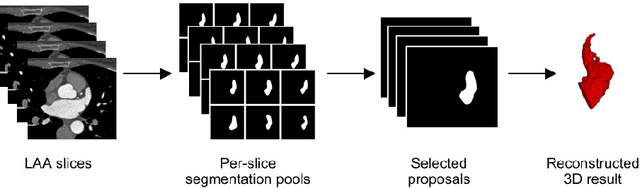
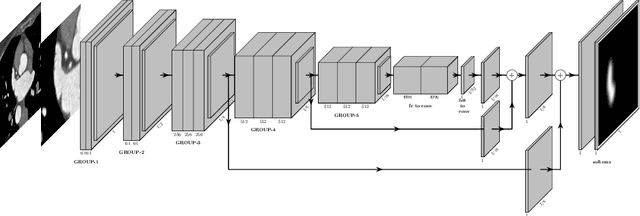
Abstract:Atrial fibrillation (AF) is a cardiovascular disease identified as one of the main risk factors for stroke. The majority of strokes due to AF are caused by clots originating in the left atrial appendage (LAA). LAA occlusion is an effective procedure for reducing stroke risk. Planning the procedure using pre-procedural imaging and analysis has shown benefits. The analysis is commonly done by manually segmenting the appendage on 2D slices. Automatic LAA segmentation methods could save an expert's time and provide insightful 3D visualizations and accurate automatic measurements to aid in medical procedures. Several semi- and fully-automatic methods for segmenting the appendage have been proposed. This paper provides a review of automatic LAA segmentation methods on 3D and 4D medical images, including CT, MRI, and echocardiogram images. We classify methods into heuristic and model-based methods, as well as into semi- and fully-automatic methods. We summarize and compare the proposed methods, evaluate their effectiveness, and present current challenges in the field and approaches to overcome them.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge