César Martínez
Orthogonal Ensemble Networks for Biomedical Image Segmentation
May 22, 2021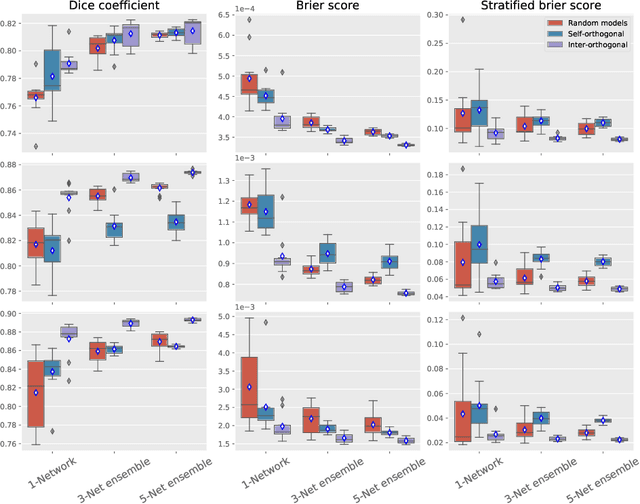
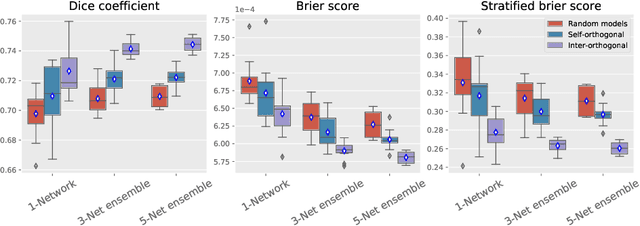
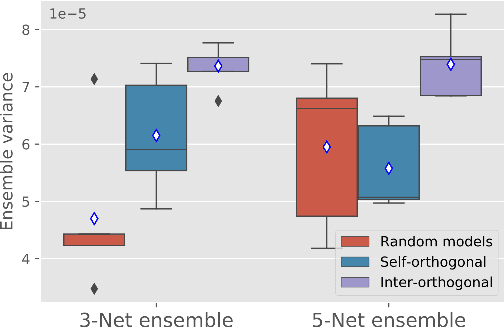
Abstract:Despite the astonishing performance of deep-learning based approaches for visual tasks such as semantic segmentation, they are known to produce miscalibrated predictions, which could be harmful for critical decision-making processes. Ensemble learning has shown to not only boost the performance of individual models but also reduce their miscalibration by averaging independent predictions. In this scenario, model diversity has become a key factor, which facilitates individual models converging to different functional solutions. In this work, we introduce Orthogonal Ensemble Networks (OEN), a novel framework to explicitly enforce model diversity by means of orthogonal constraints. The proposed method is based on the hypothesis that inducing orthogonality among the constituents of the ensemble will increase the overall model diversity. We resort to a new pairwise orthogonality constraint which can be used to regularize a sequential ensemble training process, resulting on improved predictive performance and better calibrated model outputs. We benchmark the proposed framework in two challenging brain lesion segmentation tasks --brain tumor and white matter hyper-intensity segmentation in MR images. The experimental results show that our approach produces more robust and well-calibrated ensemble models and can deal with challenging tasks in the context of biomedical image segmentation.
Post-DAE: Anatomically Plausible Segmentation via Post-Processing with Denoising Autoencoders
Jun 24, 2020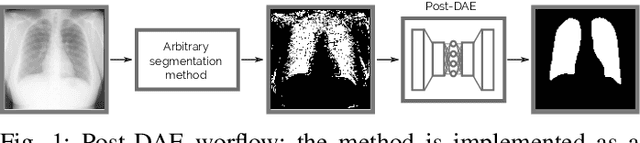
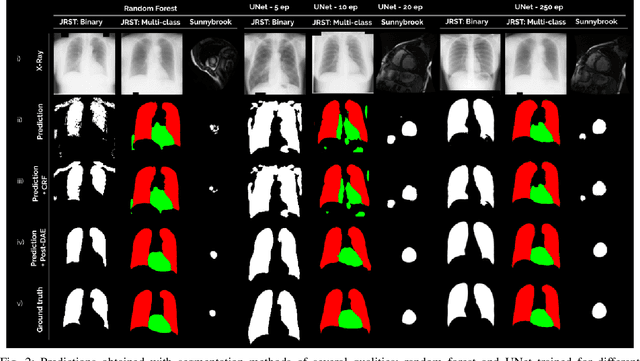
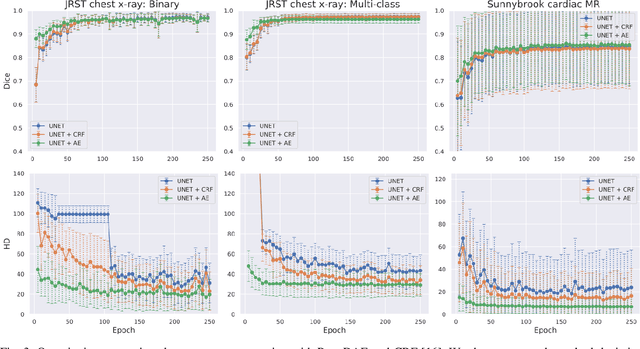
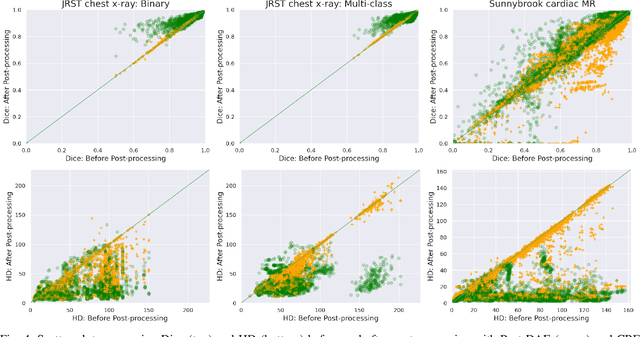
Abstract:We introduce Post-DAE, a post-processing method based on denoising autoencoders (DAE) to improve the anatomical plausibility of arbitrary biomedical image segmentation algorithms. Some of the most popular segmentation methods (e.g. based on convolutional neural networks or random forest classifiers) incorporate additional post-processing steps to ensure that the resulting masks fulfill expected connectivity constraints. These methods operate under the hypothesis that contiguous pixels with similar aspect should belong to the same class. Even if valid in general, this assumption does not consider more complex priors like topological restrictions or convexity, which cannot be easily incorporated into these methods. Post-DAE leverages the latest developments in manifold learning via denoising autoencoders. First, we learn a compact and non-linear embedding that represents the space of anatomically plausible segmentations. Then, given a segmentation mask obtained with an arbitrary method, we reconstruct its anatomically plausible version by projecting it onto the learnt manifold. The proposed method is trained using unpaired segmentation mask, what makes it independent of intensity information and image modality. We performed experiments in binary and multi-label segmentation of chest X-ray and cardiac magnetic resonance images. We show how erroneous and noisy segmentation masks can be improved using Post-DAE. With almost no additional computation cost, our method brings erroneous segmentations back to a feasible space.
* Accepted for publication in IEEE Transactions on Medical Imaging (IEEE TMI)
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge