Anna Gogolińska
Glucagon and insulin production in pancreatic cells modeled using Petri nets and Boolean networks
Apr 30, 2025



Abstract:Diabetes is a civilization chronic disease characterized by a constant elevated concentration of glucose in the blood. Many processes are involved in the glucose regulation, and their interactions are very complex. To better understand those processes we set ourselves a goal to create a Petri net model of the glucose regulation in the whole body. So far we have managed to create a model of glycolysis and synthesis of glucose in the liver, and the general overview models of the glucose regulation in a healthy and diabetic person. In this paper we introduce Petri nets models of insulin secretion in beta cell of the pancreas, and glucagon in the pancreas alpha cells. Those two hormones have mutually opposite effects: insulin preventing hyperglycemia, and glucagon preventing hypoglycemia. Understanding the mechanisms of insulin and glucagon secretion constitutes the basis for understanding diabetes. We also present a model in which both processes occur together, depending on the blood glucose level. The dynamics of each model is analysed. Additionally, we transform the overall insulin and glucagon secretion system to a Boolean network, following standard transformation rules.
Petri nets in modelling glucose regulating processes in the liver
May 17, 2024Abstract:Diabetes is a chronic condition, considered one of the civilization diseases, that is characterized by sustained high blood sugar levels. There is no doubt that more and more people is going to suffer from diabetes, hence it is crucial to understand better its biological foundations. The essential processes related to the control of glucose levels in the blood are: glycolysis (process of breaking down of glucose) and glucose synthesis, both taking place in the liver. The glycolysis occurs during feeding and it is stimulated by insulin. On the other hand, the glucose synthesis arises during fasting and it is stimulated by glucagon. In the paper we present a Petri net model of glycolysis and glucose synthesis in the liver. The model is created based on medical literature. Standard Petri nets techniques are used to analyse the properties of the model: traps, reachability graphs, tokens dynamics, deadlocks analysis. The results are described in the paper. Our analysis shows that the model captures the interactions between different enzymes and substances, which is consistent with the biological processes occurring during fasting and feeding. The model constitutes the first element of our long-time goal to create the whole body model of the glucose regulation in a healthy human and a person with diabetes.
Acyclic and Cyclic Reversing Computations in Petri Nets
Aug 04, 2021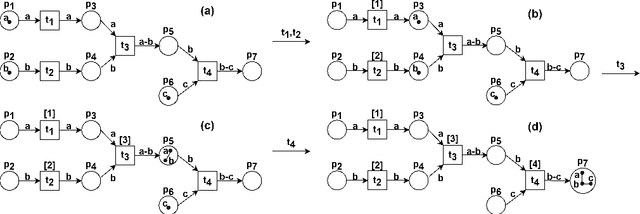
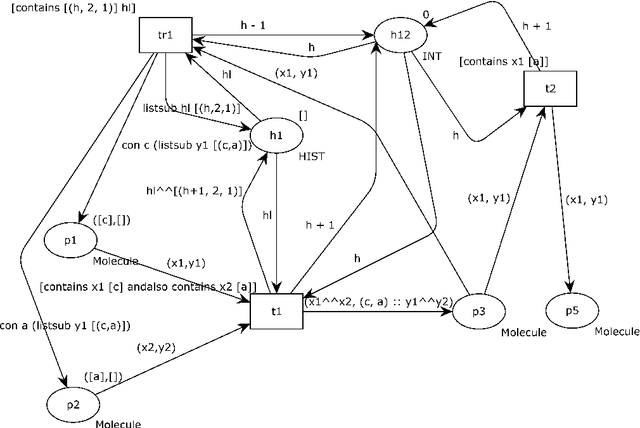
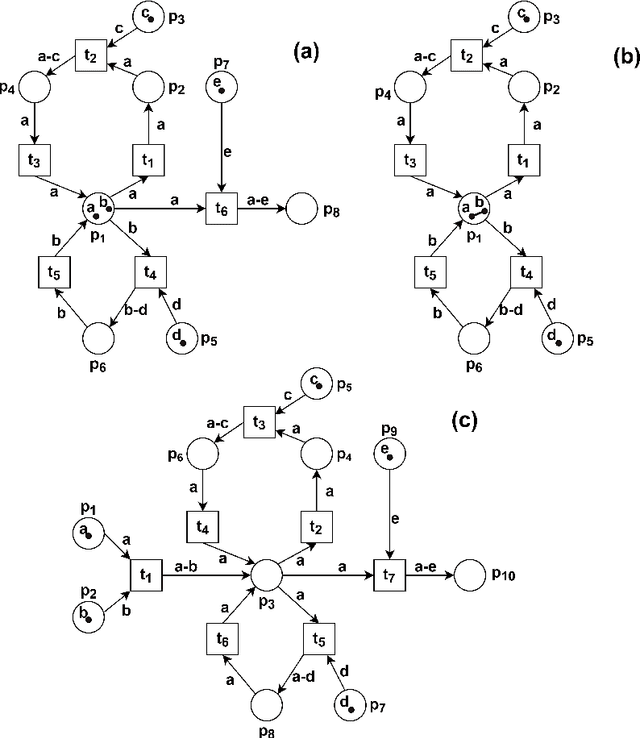
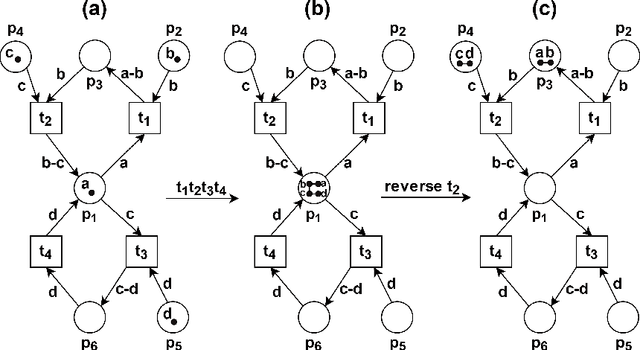
Abstract:Reversible computations constitute an unconventional form of computing where any sequence of performed operations can be undone by executing in reverse order at any point during a computation. It has been attracting increasing attention as it provides opportunities for low-power computation, being at the same time essential or eligible in various applications. In recent work, we have proposed a structural way of translating Reversing Petri Nets (RPNs) - a type of Petri nets that embeds reversible computation, to bounded Coloured Petri Nets (CPNs) - an extension of traditional Petri Nets, where tokens carry data values. Three reversing semantics are possible in RPNs: backtracking (reversing of the lately executed action), causal reversing (action can be reversed only when all its effects have been undone) and out of causal reversing (any previously performed action can be reversed). In this paper, we extend the RPN to CPN translation with formal proofs of correctness. Moreover, the possibility of introduction of cycles to RPNs is discussed. We analyze which type of cycles could be allowed in RPNs to ensure consistency with the current semantics. It emerged that the most interesting case related to cycles in RPNs occurs in causal semantics, where various interpretations of dependency result in different net's behaviour during reversing. Three definitions of dependence are presented and discussed.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge