Alessandro Veneziani
Physics Guided Machine Learning for Variational Multiscale Reduced Order Modeling
May 25, 2022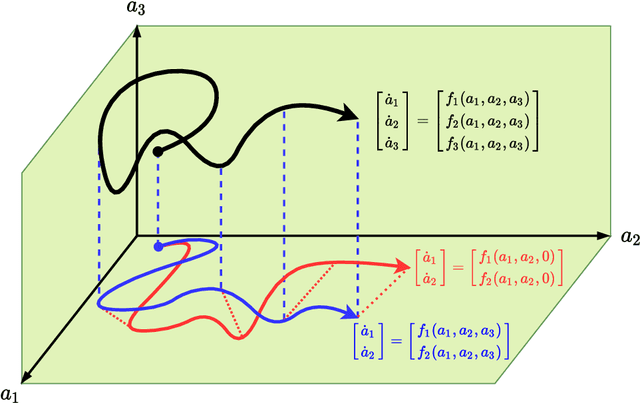
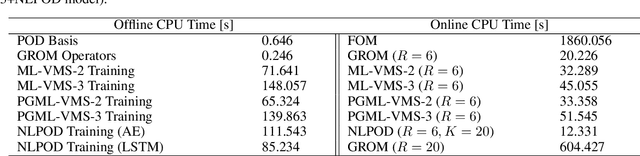
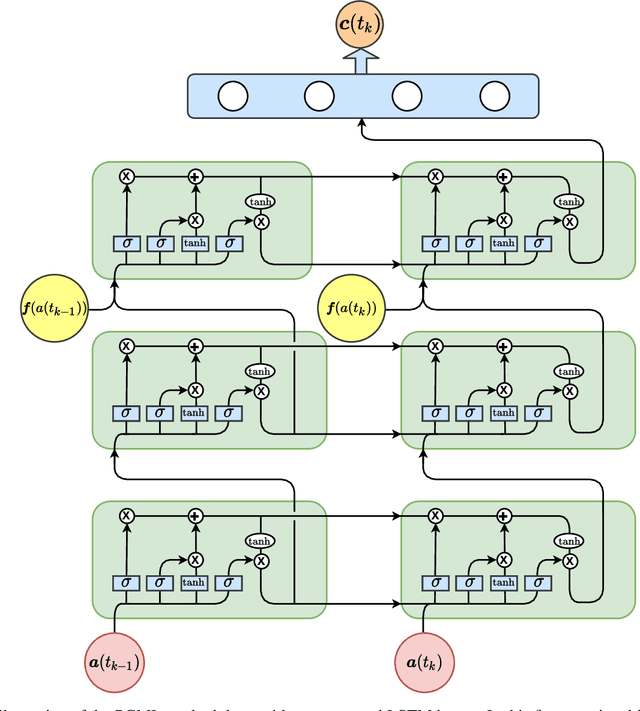
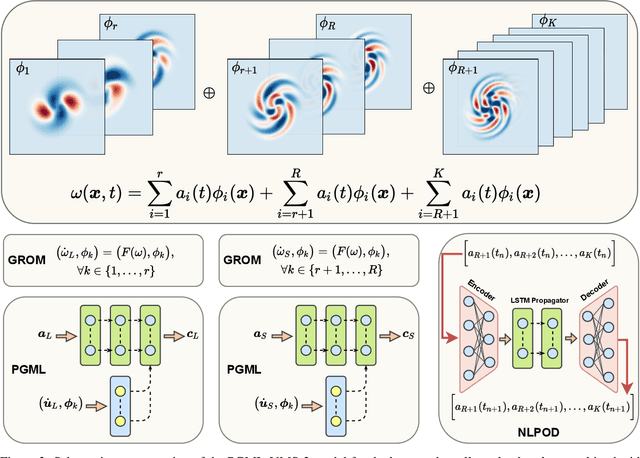
Abstract:We propose a new physics guided machine learning (PGML) paradigm that leverages the variational multiscale (VMS) framework and available data to dramatically increase the accuracy of reduced order models (ROMs) at a modest computational cost. The hierarchical structure of the ROM basis and the VMS framework enable a natural separation of the resolved and unresolved ROM spatial scales. Modern PGML algorithms are used to construct novel models for the interaction among the resolved and unresolved ROM scales. Specifically, the new framework builds ROM operators that are closest to the true interaction terms in the VMS framework. Finally, machine learning is used to reduce the projection error and further increase the ROM accuracy. Our numerical experiments for a two-dimensional vorticity transport problem show that the novel PGML-VMS-ROM paradigm maintains the low computational cost of current ROMs, while significantly increasing the ROM accuracy.
Patient-Specific 3D Volumetric Reconstruction of Bioresorbable Stents: A Method to Generate 3D Geometries for Computational Analysis of Coronaries Treated with Bioresorbable Stents
Oct 08, 2018



Abstract:As experts continue to debate the optimal surgery practice for coronary disease - percutaneous coronary intervention (PCI) or coronary aortic bypass graft (CABG) - computational tools may provide a quantitative assessment of each option. Computational fluid dynamics (CFD) has been used to assess the interplay between hemodynamics and stent struts; it is of particular interest in Bioresorbable Vascular Stents (BVS), since their thicker struts may result in impacted flow patterns and possible pathological consequences. Many proofs of concept are presented in the literature; however, a practical method for extracting patient-specific stented coronary artery geometries from images over a large number of patients remains an open problem. This work provides a possible pipeline for the reconstruction of the BVS. Using Optical Coherence Tomographies (OCT) and Invasive Coronary Angiographies (ICA), we can reconstruct the 3D geometry of deployed BVS in vivo. We illustrate the stent reconstruction process: (i) automatic strut detection, (ii) identification of stent components, (iii) 3D registration of stent curvature, and (iv) final stent volume reconstruction. The methodology is designed for use on clinical OCT images, as opposed to approaches that relied on a small number of virtually deployed stents. The proposed reconstruction process is validated with a virtual phantom stent, providing quantitative assessment of the methodology, and with selected clinical cases, confirming feasibility. Using multimodality image analysis, we obtain reliable reconstructions within a reasonable timeframe. This work is the first step toward a fully automated reconstruction and simulation procedure aiming at an extensive quantitative analysis of the impact of BVS struts on hemodynamics via CFD in clinical trials, going beyond the proof-of-concept stage.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge