Tom Edinburgh
Clinical characteristics, complications and outcomes of critically ill patients with Dengue in Brazil, 2012-2024: a nationwide, multicentre cohort study
Aug 25, 2025Abstract:Background. Dengue outbreaks are a major public health issue, with Brazil reporting 71% of global cases in 2024. Purpose. This study aims to describe the profile of severe dengue patients admitted to Brazilian Intensive Care units (ICUs) (2012-2024), assess trends over time, describe new onset complications while in ICU and determine the risk factors at admission to develop complications during ICU stay. Methods. We performed a prospective study of dengue patients from 253 ICUs across 56 hospitals. We used descriptive statistics to describe the dengue ICU population, logistic regression to identify risk factors for complications during the ICU stay, and a machine learning framework to predict the risk of evolving to complications. Visualisations were generated using ISARIC VERTEX. Results. Of 11,047 admissions, 1,117 admissions (10.1%) evolved to complications, including non-invasive (437 admissions) and invasive ventilation (166), vasopressor (364), blood transfusion (353) and renal replacement therapy (103). Age>80 (OR: 3.10, 95% CI: 2.02-4.92), chronic kidney disease (OR: 2.94, 2.22-3.89), liver cirrhosis (OR: 3.65, 1.82-7.04), low platelets (<50,000 cells/mm3; OR: OR: 2.25, 1.89-2.68), and high leukocytes (>7,000 cells/mm3; OR: 2.47, 2.02-3.03) were significant risk factors for complications. A machine learning tool for predicting complications was proposed, showing accurate discrimination and calibration. Conclusion. We described a large cohort of dengue patients admitted to ICUs and identified key risk factors for severe dengue complications, such as advanced age, presence of comorbidities, higher level of leukocytes and lower level of platelets. The proposed prediction tool can be used for early identification and targeted interventions to improve outcomes in dengue-endemic regions.
DeepClean -- self-supervised artefact rejection for intensive care waveform data using generative deep learning
Sep 05, 2019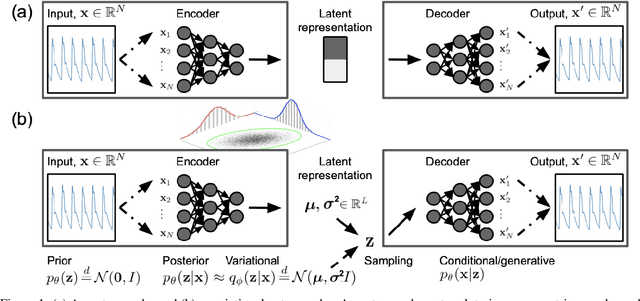
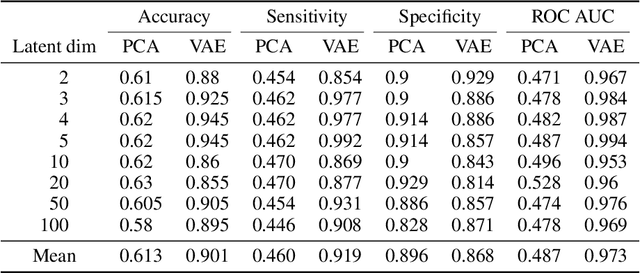
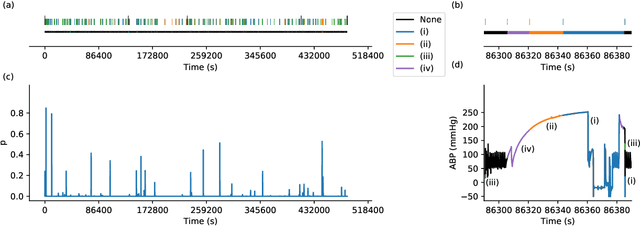
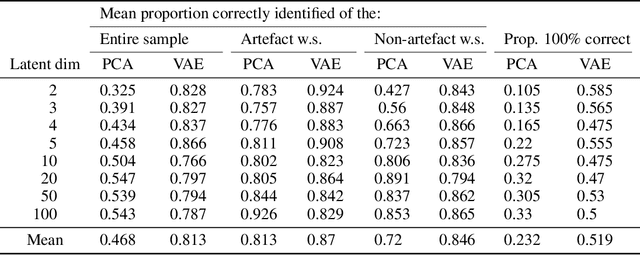
Abstract:Waveform physiological data is important in the treatment of critically ill patients in the intensive care unit. Such recordings are susceptible to artefacts, which must be removed before the data can be re-used for alerting or reprocessed for other clinical or research purposes. Accurate removal of artefacts reduces both bias and uncertainty in clinical assessment and the false positive rate of intensive care unit alarms, and is therefore a key component in providing optimal clinical care. In this work, we present DeepClean; a prototype self-supervised artefact detection system using a convolutional variational autoencoder deep neural network that avoids costly and painstaking manual annotation, requiring only easily-obtained 'good' data for training. For a test case with invasive arterial blood pressure, we demonstrate that our algorithm can detect the presence of an artefact within a 10-second sample of data with sensitivity and specificity around 90%. Furthermore, DeepClean was able to identify regions of artefact within such samples with high accuracy and we show that it significantly outperforms a baseline principle component analysis approach in both signal reconstruction and artefact detection. DeepClean learns a generative model and therefore may also be used for imputation of missing data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge