Nadezhda Alsahanova
Integrating Radiomics with Deep Learning Enhances Multiple Sclerosis Lesion Delineation
Jun 17, 2025Abstract:Background: Accurate lesion segmentation is critical for multiple sclerosis (MS) diagnosis, yet current deep learning approaches face robustness challenges. Aim: This study improves MS lesion segmentation by combining data fusion and deep learning techniques. Materials and Methods: We suggested novel radiomic features (concentration rate and R\'enyi entropy) to characterize different MS lesion types and fused these with raw imaging data. The study integrated radiomic features with imaging data through a ResNeXt-UNet architecture and attention-augmented U-Net architecture. Our approach was evaluated on scans from 46 patients (1102 slices), comparing performance before and after data fusion. Results: The radiomics-enhanced ResNeXt-UNet demonstrated high segmentation accuracy, achieving significant improvements in precision and sensitivity over the MRI-only baseline and a Dice score of 0.774$\pm$0.05; p<0.001 according to Bonferroni-adjusted Wilcoxon signed-rank tests. The radiomics-enhanced attention-augmented U-Net model showed a greater model stability evidenced by reduced performance variability (SDD = 0.18 $\pm$ 0.09 vs. 0.21 $\pm$ 0.06; p=0.03) and smoother validation curves with radiomics integration. Conclusion: These results validate our hypothesis that fusing radiomics with raw imaging data boosts segmentation performance and stability in state-of-the-art models.
Image Segmentation with Topological Priors
May 12, 2022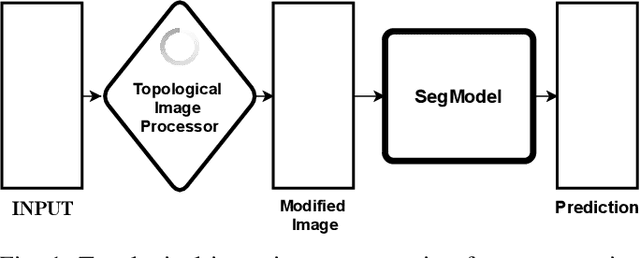
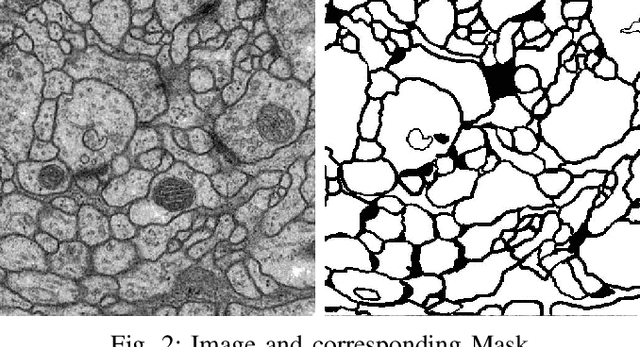
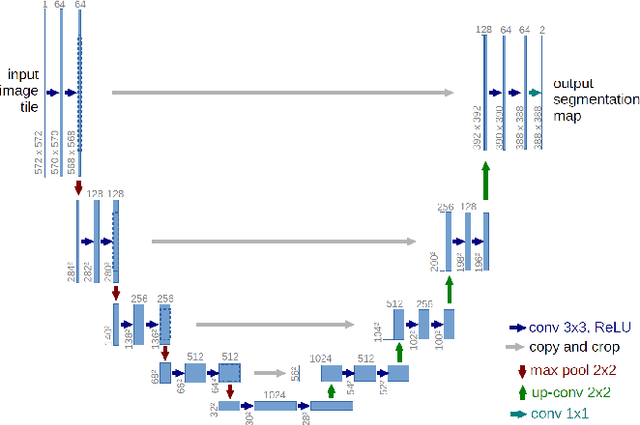
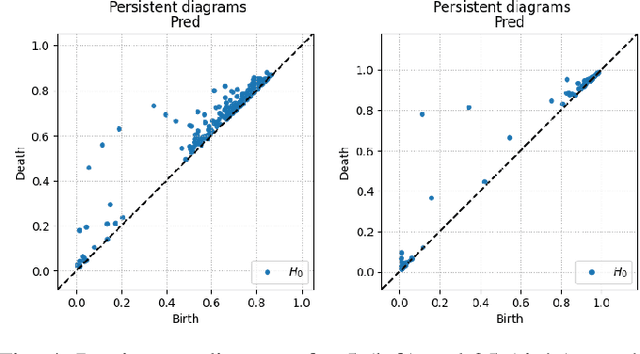
Abstract:Solving segmentation tasks with topological priors proved to make fewer errors in fine-scale structures. In this work, we use topological priors both before and during the deep neural network training procedure. We compared the results of the two approaches with simple segmentation on various accuracy metrics and the Betti number error, which is directly related to topological correctness, and discovered that incorporating topological information into the classical UNet model performed significantly better. We conducted experiments on the ISBI EM segmentation dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge