Mikael Brudfors
Joint Total Variation ESTATICS for Robust Multi-Parameter Mapping
May 28, 2020
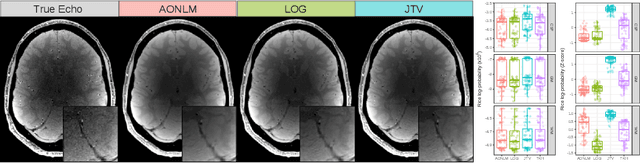
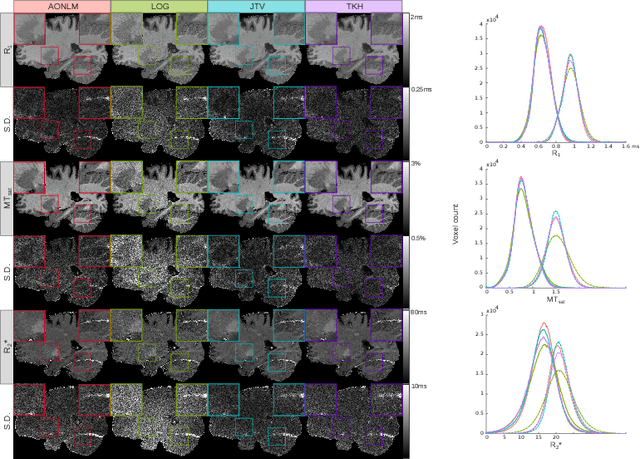
Abstract:Quantitative magnetic resonance imaging (qMRI) derives tissue-specific parameters -- such as the apparent transverse relaxation rate R2*, the longitudinal relaxation rate R1 and the magnetisation transfer saturation -- that can be compared across sites and scanners and carry important information about the underlying microstructure. The multi-parameter mapping (MPM) protocol takes advantage of multi-echo acquisitions with variable flip angles to extract these parameters in a clinically acceptable scan time. In this context, ESTATICS performs a joint loglinear fit of multiple echo series to extract R2* and multiple extrapolated intercepts, thereby improving robustness to motion and decreasing the variance of the estimators. In this paper, we extend this model in two ways: (1) by introducing a joint total variation (JTV) prior on the intercepts and decay, and (2) by deriving a nonlinear maximum \emph{a posteriori} estimate. We evaluated the proposed algorithm by predicting left-out echoes in a rich single-subject dataset. In this validation, we outperformed other state-of-the-art methods and additionally showed that the proposed approach greatly reduces the variance of the estimated maps, without introducing bias.
Groupwise Multimodal Image Registration using Joint Total Variation
May 06, 2020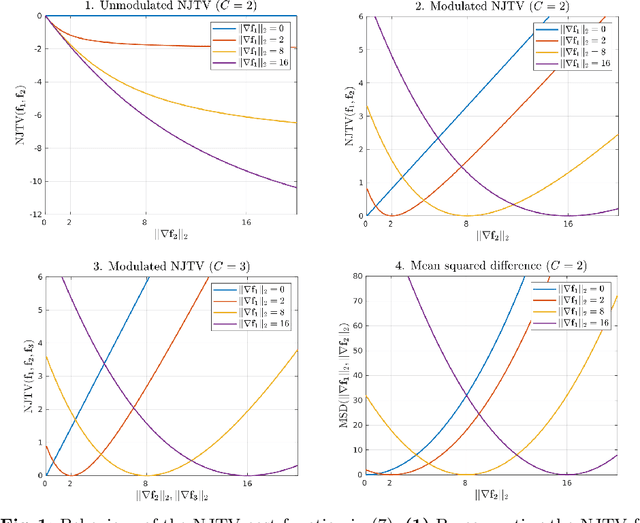
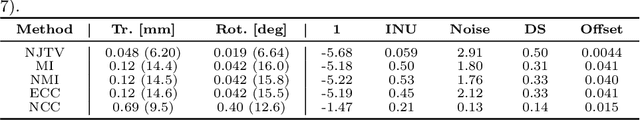
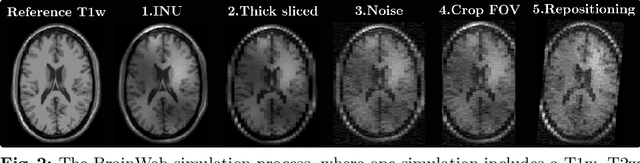
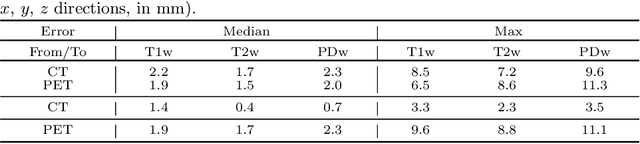
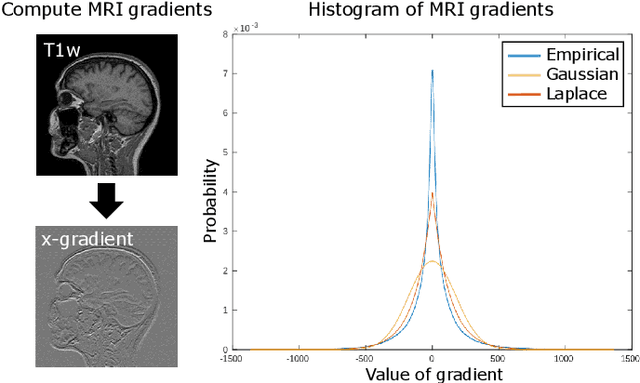
Abstract:In medical imaging it is common practice to acquire a wide range of modalities (MRI, CT, PET, etc.), to highlight different structures or pathologies. As patient movement between scans or scanning session is unavoidable, registration is often an essential step before any subsequent image analysis. In this paper, we introduce a cost function based on joint total variation for such multimodal image registration. This cost function has the advantage of enabling principled, groupwise alignment of multiple images, whilst being insensitive to strong intensity non-uniformities. We evaluate our algorithm on rigidly aligning both simulated and real 3D brain scans. This validation shows robustness to strong intensity non-uniformities and low registration errors for CT/PET to MRI alignment. Our implementation is publicly available at https://github.com/brudfors/coregistration-njtv.
A Tool for Super-Resolving Multimodal Clinical MRI
Sep 03, 2019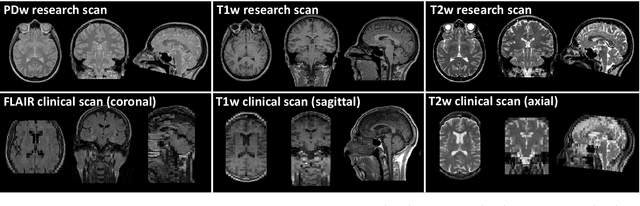

Abstract:We present a tool for resolution recovery in multimodal clinical magnetic resonance imaging (MRI). Such images exhibit great variability, both biological and instrumental. This variability makes automated processing with neuroimaging analysis software very challenging. This leaves intelligence extractable only from large-scale analyses of clinical data untapped, and impedes the introduction of automated predictive systems in clinical care. The tool presented in this paper enables such processing, via inference in a generative model of thick-sliced, multi-contrast MR scans. All model parameters are estimated from the observed data, without the need for manual tuning. The model-driven nature of the approach means that no type of training is needed for applicability to the diversity of MR contrasts present in a clinical context. We show on simulated data that the proposed approach outperforms conventional model-based techniques, and on a large hospital dataset of multimodal MRIs that the tool can successfully super-resolve very thick-sliced images. The implementation is available from https://github.com/brudfors/spm_superres.
Empirical Bayesian Mixture Models for Medical Image Translation
Aug 16, 2019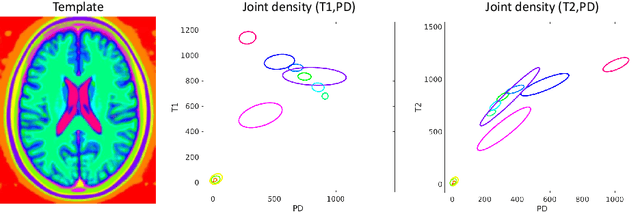

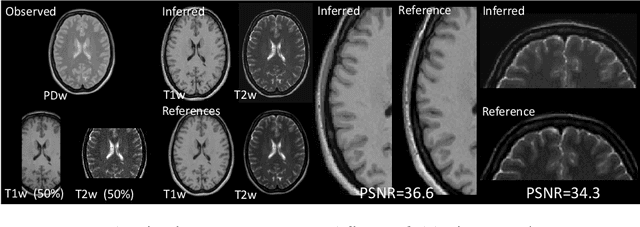
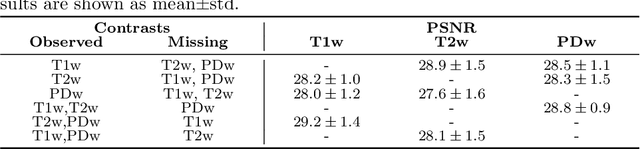
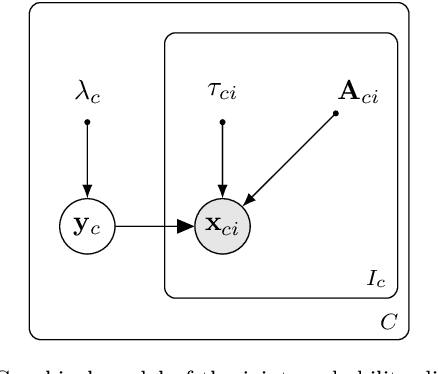
Abstract:Automatically generating one medical imaging modality from another is known as medical image translation, and has numerous interesting applications. This paper presents an interpretable generative modelling approach to medical image translation. By allowing a common model for group-wise normalisation and segmentation of brain scans to handle missing data, the model allows for predicting entirely missing modalities from one, or a few, MR contrasts. Furthermore, the model can be trained on a fairly small number of subjects. The proposed model is validated on three clinically relevant scenarios. Results appear promising and show that a principled, probabilistic model of the relationship between multi-channel signal intensities can be used to infer missing modalities -- both MR contrasts and CT images.
Nonlinear Markov Random Fields Learned via Backpropagation
Mar 08, 2019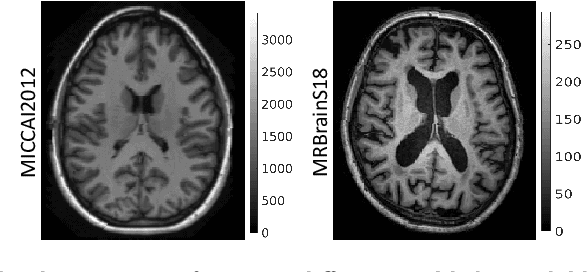
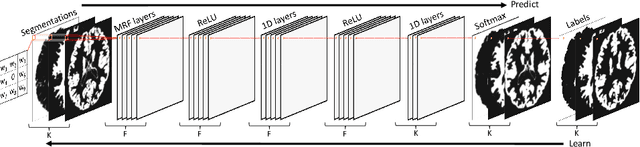
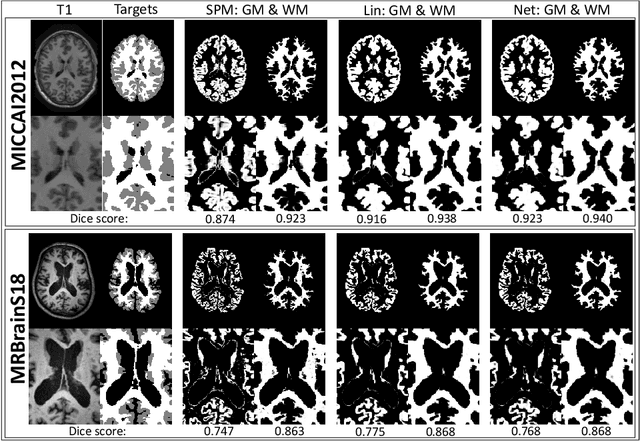
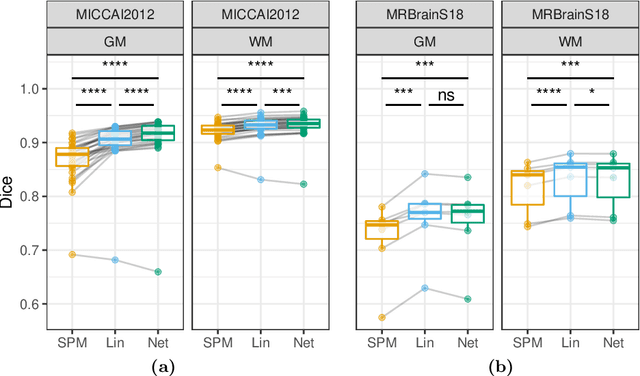
Abstract:Although convolutional neural networks (CNNs) currently dominate competitions on image segmentation, for neuroimaging analysis tasks, more classical generative approaches based on mixture models are still used in practice to parcellate brains. To bridge the gap between the two, in this paper we propose a marriage between a probabilistic generative model, which has been shown to be robust to variability among magnetic resonance (MR) images acquired via different imaging protocols, and a CNN. The link is in the prior distribution over the unknown tissue classes, which are classically modelled using a Markov random field. In this work we model the interactions among neighbouring pixels by a type of recurrent CNN, which can encode more complex spatial interactions. We validate our proposed model on publicly available MR data, from different centres, and show that it generalises across imaging protocols. This result demonstrates a successful and principled inclusion of a CNN in a generative model, which in turn could be adapted by any probabilistic generative approach for image segmentation.
MRI Super-Resolution using Multi-Channel Total Variation
Oct 18, 2018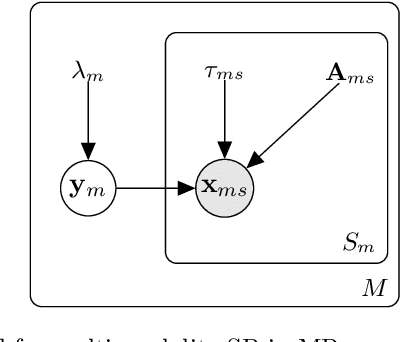


Abstract:This paper presents a generative model for super-resolution in routine clinical magnetic resonance images (MRI), of arbitrary orientation and contrast. The model recasts the recovery of high resolution images as an inverse problem, in which a forward model simulates the slice-select profile of the MR scanner. The paper introduces a prior based on multi-channel total variation for MRI super-resolution. Bias-variance trade-off is handled by estimating hyper-parameters from the low resolution input scans. The model was validated on a large database of brain images. The validation showed that the model can improve brain segmentation, that it can recover anatomical information between images of different MR contrasts, and that it generalises well to the large variability present in MR images of different subjects. The implementation is freely available at https://github.com/WCHN/mtv-preproc
An Algorithm for Learning Shape and Appearance Models without Annotations
Jul 27, 2018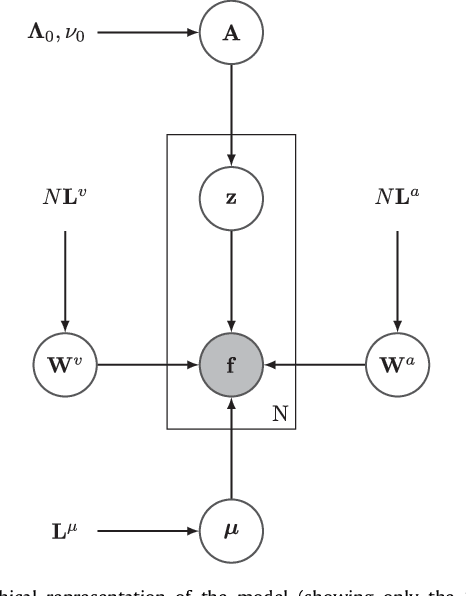
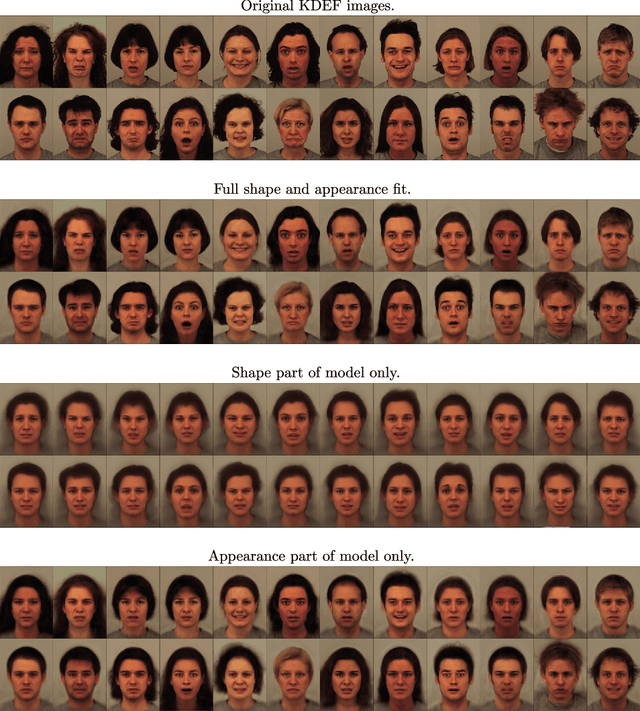


Abstract:This paper presents a framework for automatically learning shape and appearance models for medical (and certain other) images. It is based on the idea that having a more accurate shape and appearance model leads to more accurate image registration, which in turn leads to a more accurate shape and appearance model. This leads naturally to an iterative scheme, which is based on a probabilistic generative model that is fit using Gauss-Newton updates within an EM-like framework. It was developed with the aim of enabling distributed privacy-preserving analysis of brain image data, such that shared information (shape and appearance basis functions) may be passed across sites, whereas latent variables that encode individual images remain secure within each site. These latent variables are proposed as features for privacy-preserving data mining applications. The approach is demonstrated qualitatively on the KDEF dataset of 2D face images, showing that it can align images that traditionally require shape and appearance models trained using manually annotated data (manually defined landmarks etc.). It is applied to MNIST dataset of handwritten digits to show its potential for machine learning applications, particularly when training data is limited. The model is able to handle ``missing data'', which allows it to be cross-validated according to how well it can predict left-out voxels. The suitability of the derived features for classifying individuals into patient groups was assessed by applying it to a dataset of over 1,900 segmented T1-weighted MR images, which included images from the COBRE and ABIDE datasets.
Diffeomorphic brain shape modelling using Gauss-Newton optimisation
Jun 19, 2018
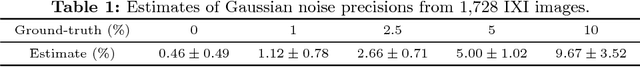
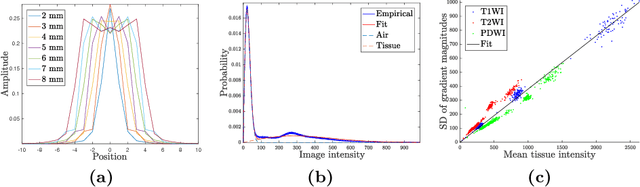

Abstract:Shape modelling describes methods aimed at capturing the natural variability of shapes and commonly relies on probabilistic interpretations of dimensionality reduction techniques such as principal component analysis. Due to their computational complexity when dealing with dense deformation models such as diffeomorphisms, previous attempts have focused on explicitly reducing their dimension, diminishing de facto their flexibility and ability to model complex shapes such as brains. In this paper, we present a generative model of shape that allows the covariance structure of deformations to be captured without squashing their domain, resulting in better normalisation. An efficient inference scheme based on Gauss-Newton optimisation is used, which enables processing of 3D neuroimaging data. We trained this algorithm on segmented brains from the OASIS database, generating physiologically meaningful deformation trajectories. To prove the model's robustness, we applied it to unseen data, which resulted in equivalent fitting scores.
* 8 pages, 4 figures, conference paper, accepted at MICCAI 2018
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge