Konstantinos Zormpas-Petridis
Generalizing imaging biomarker repeatability studies using Bayesian inference: Applications in detecting heterogeneous treatment response in whole-body diffusion-weighted MRI of metastatic prostate cancer
May 14, 2025Abstract:The assessment of imaging biomarkers is critical for advancing precision medicine and improving disease characterization. Despite the availability of methods to derive disease heterogeneity metrics in imaging studies, a robust framework for evaluating measurement uncertainty remains underdeveloped. To address this gap, we propose a novel Bayesian framework to assess the precision of disease heterogeneity measures in biomarker studies. Our approach extends traditional methods for evaluating biomarker precision by providing greater flexibility in statistical assumptions and enabling the analysis of biomarkers beyond univariate or multivariate normally-distributed variables. Using Hamiltonian Monte Carlo sampling, the framework supports both, for example, normally-distributed and Dirichlet-Multinomial distributed variables, enabling the derivation of posterior distributions for biomarker parameters under diverse model assumptions. Designed to be broadly applicable across various imaging modalities and biomarker types, the framework builds a foundation for generalizing reproducible and objective biomarker evaluation. To demonstrate utility, we apply the framework to whole-body diffusion-weighted MRI (WBDWI) to assess heterogeneous therapeutic responses in metastatic bone disease. Specifically, we analyze data from two patient studies investigating treatments for metastatic castrate-resistant prostate cancer (mCRPC). Our results reveal an approximately 70% response rate among individual tumors across both studies, objectively characterizing differential responses to systemic therapies and validating the clinical relevance of the proposed methodology. This Bayesian framework provides a powerful tool for advancing biomarker research across diverse imaging-based studies while offering valuable insights into specific clinical applications, such as mCRPC treatment response.
Capturing global spatial context for accurate cell classification in skin cancer histology
Aug 07, 2018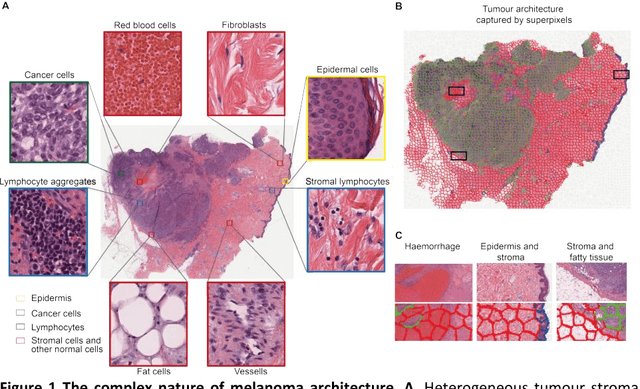
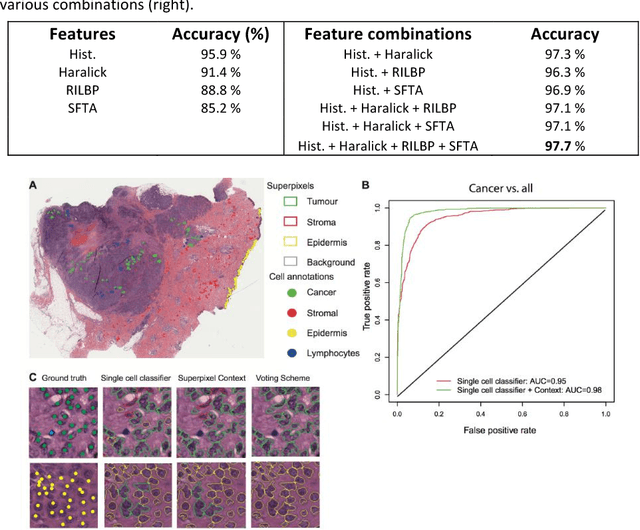
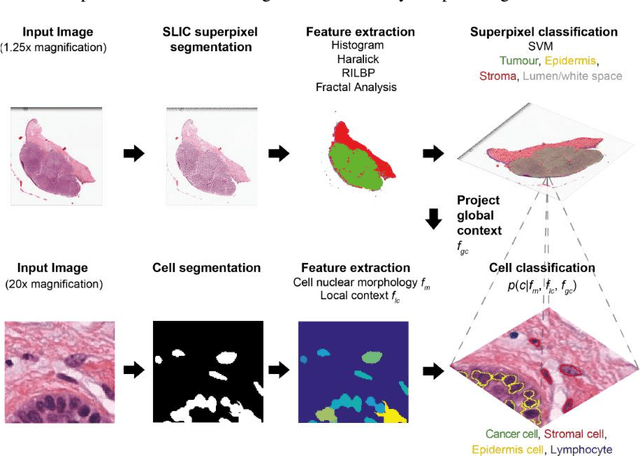
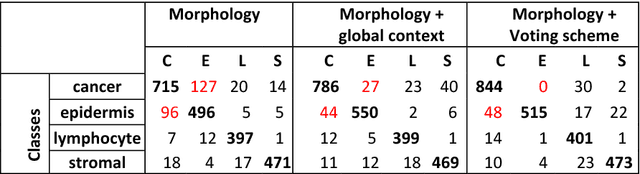
Abstract:The spectacular response observed in clinical trials of immunotherapy in patients with previously uncurable Melanoma, a highly aggressive form of skin cancer, calls for a better understanding of the cancer-immune interface. Computational pathology provides a unique opportunity to spatially dissect such interface on digitised pathological slides. Accurate cellular classification is a key to ensure meaningful results, but is often challenging even with state-of-art machine learning and deep learning methods. We propose a hierarchical framework, which mirrors the way pathologists perceive tumour architecture and define tumour heterogeneity to improve cell classification methods that rely solely on cell nuclei morphology. The SLIC superpixel algorithm was used to segment and classify tumour regions in low resolution H&E-stained histological images of melanoma skin cancer to provide a global context. Classification of superpixels into tumour, stroma, epidermis and lumen/white space, yielded a 97.7% training set accuracy and 95.7% testing set accuracy in 58 whole-tumour images of the TCGA melanoma dataset. The superpixel classification was projected down to high resolution images to enhance the performance of a single cell classifier, based on cell nuclear morphological features, and resulted in increasing its accuracy from 86.4% to 91.6%. Furthermore, a voting scheme was proposed to use global context as biological a priori knowledge, pushing the accuracy further to 92.8%. This study demonstrates how using the global spatial context can accurately characterise the tumour microenvironment and allow us to extend significantly beyond single-cell morphological classification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge