Herve Hugonnet
Single-shot refractive index slice imaging using spectrally multiplexed optical transfer function reshaping
Jan 13, 2023



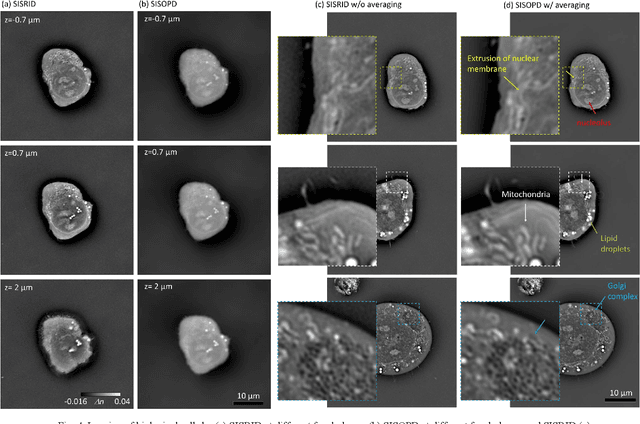
Abstract:The refractive index (RI) of cells and tissues is crucial in pathophysiology as a noninvasive and quantitative imaging contrast. Although its measurements have been demonstrated using three-dimensional quantitative phase imaging methods, these methods often require bulky interferometric setups or multiple measurements, which limits the measurement sensitivity and speed. Here, we present a single-shot RI imaging method that visualizes the RI of the in-focus region of a sample. By exploiting spectral multiplexing and optical transfer function engineering, three color-coded intensity images of a sample with three optimized illuminations were simultaneously obtained in a single-shot measurement. The measured intensity images were then deconvoluted to obtain the RI image of the in-focus slice of the sample. As a proof of concept, a setup was built using Fresnel lenses and a liquid-crystal display. For validation purposes, we measured microspheres of known RI and cross-validated the results with simulated results. Various static and highly dynamic biological cells were imaged to demonstrate that the proposed method can conduct single-shot RI slice imaging of biological samples with subcellular resolution.
Quantitative phase and refractive index imaging of 3D objects via optical transfer function reshaping
Jan 21, 2022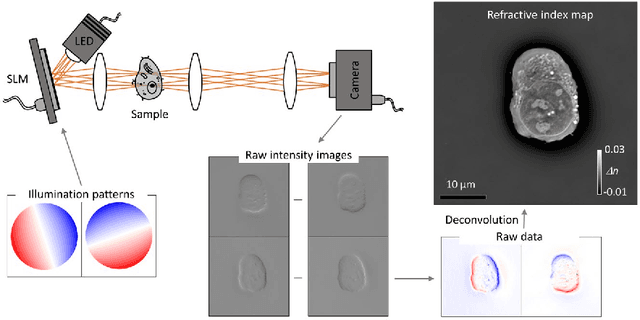
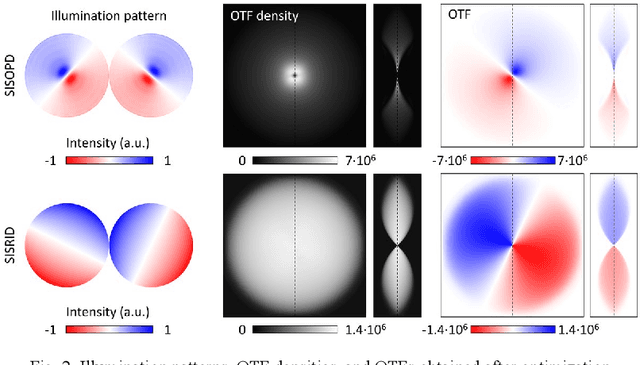
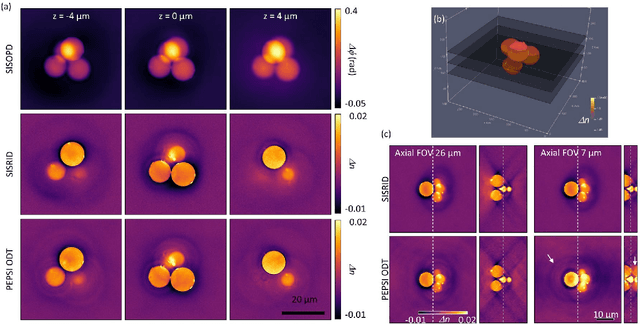
Abstract:Deconvolution phase microscopy enables high-contrast visualization of transparent samples through reconstructions of their transmitted phases or refractive indexes. Herein, we propose a method to extend 2D deconvolution phase microscopy to thick 3D samples. The refractive index distribution of a sample can be obtained at a specific axial plane by measuring only four intensity images obtained under optimized illumination patterns. Also, the optical phase delay of a sample can be measured using different illumination patterns.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge