Frederick H. Streitz
Machine Learning-driven Multiscale MD Workflows: The Mini-MuMMI Experience
Jul 10, 2025Abstract:Computational models have become one of the prevalent methods to model complex phenomena. To accurately model complex interactions, such as detailed biomolecular interactions, scientists often rely on multiscale models comprised of several internal models operating at difference scales, ranging from microscopic to macroscopic length and time scales. Bridging the gap between different time and length scales has historically been challenging but the advent of newer machine learning (ML) approaches has shown promise for tackling that task. Multiscale models require massive amounts of computational power and a powerful workflow management system. Orchestrating ML-driven multiscale studies on parallel systems with thousands of nodes is challenging, the workflow must schedule, allocate and control thousands of simulations operating at different scales. Here, we discuss the massively parallel Multiscale Machine-Learned Modeling Infrastructure (MuMMI), a multiscale workflow management infrastructure, that can orchestrate thousands of molecular dynamics (MD) simulations operating at different timescales, spanning from millisecond to nanosecond. More specifically, we introduce a novel version of MuMMI called "mini-MuMMI". Mini-MuMMI is a curated version of MuMMI designed to run on modest HPC systems or even laptops whereas MuMMI requires larger HPC systems. We demonstrate mini-MuMMI utility by exploring RAS-RAF membrane interactions and discuss the different challenges behind the generalization of multiscale workflows and how mini-MuMMI can be leveraged to target a broader range of applications outside of MD and RAS-RAF interactions.
Machine Learning-Powered Mitigation Policy Optimization in Epidemiological Models
Oct 16, 2020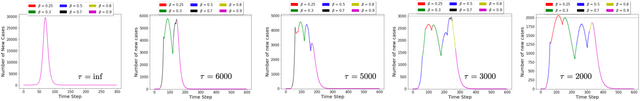
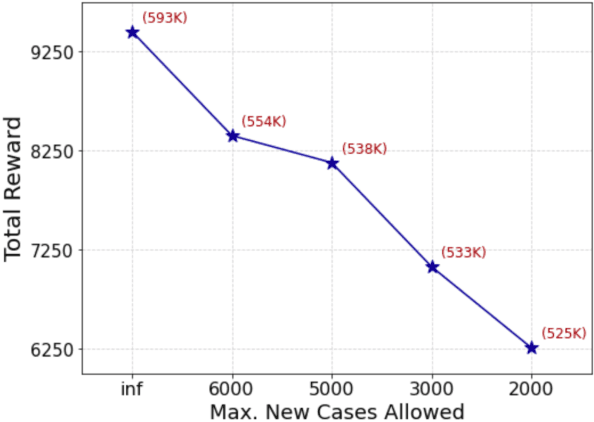
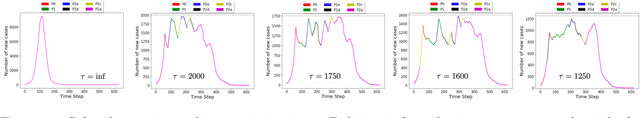
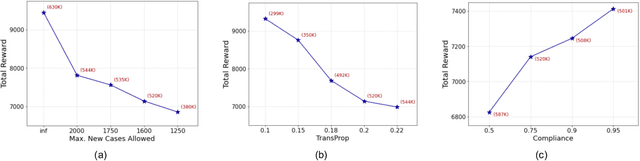
Abstract:A crucial aspect of managing a public health crisis is to effectively balance prevention and mitigation strategies, while taking their socio-economic impact into account. In particular, determining the influence of different non-pharmaceutical interventions (NPIs) on the effective use of public resources is an important problem, given the uncertainties on when a vaccine will be made available. In this paper, we propose a new approach for obtaining optimal policy recommendations based on epidemiological models, which can characterize the disease progression under different interventions, and a look-ahead reward optimization strategy to choose the suitable NPI at different stages of an epidemic. Given the time delay inherent in any epidemiological model and the exponential nature especially of an unmanaged epidemic, we find that such a look-ahead strategy infers non-trivial policies that adhere well to the constraints specified. Using two different epidemiological models, namely SEIR and EpiCast, we evaluate the proposed algorithm to determine the optimal NPI policy, under a constraint on the number of daily new cases and the primary reward being the absence of restrictions.
Accurate Calibration of Agent-based Epidemiological Models with Neural Network Surrogates
Oct 13, 2020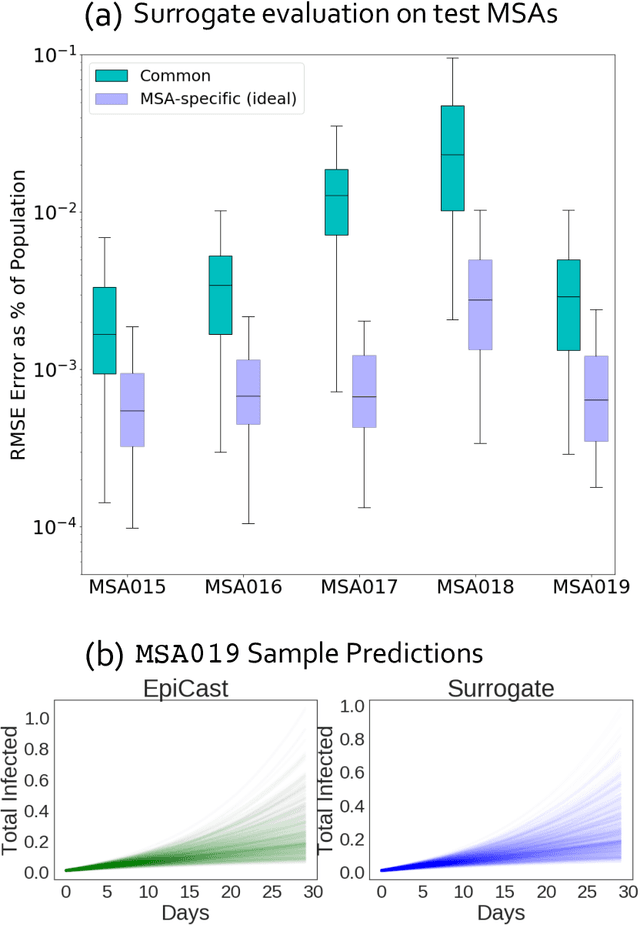
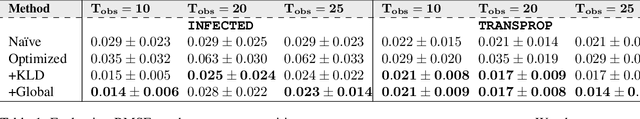
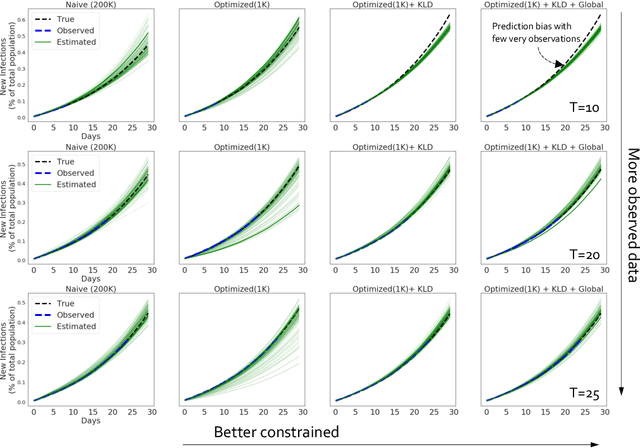
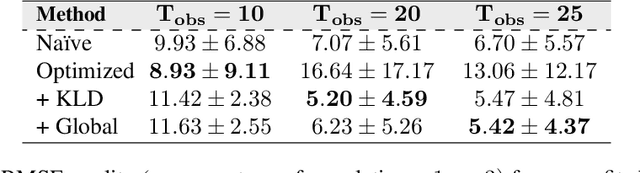
Abstract:Calibrating complex epidemiological models to observed data is a crucial step to provide both insights into the current disease dynamics, i.e.\ by estimating a reproductive number, as well as to provide reliable forecasts and scenario explorations. Here we present a new approach to calibrate an agent-based model -- EpiCast -- using a large set of simulation ensembles for different major metropolitan areas of the United States. In particular, we propose: a new neural network based surrogate model able to simultaneously emulate all different locations; and a novel posterior estimation that provides not only more accurate posterior estimates of all parameters but enables the joint fitting of global parameters across regions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge