Francis G. Spinale
Using Foundation Models as Pseudo-Label Generators for Pre-Clinical 4D Cardiac CT Segmentation
May 14, 2025Abstract:Cardiac image segmentation is an important step in many cardiac image analysis and modeling tasks such as motion tracking or simulations of cardiac mechanics. While deep learning has greatly advanced segmentation in clinical settings, there is limited work on pre-clinical imaging, notably in porcine models, which are often used due to their anatomical and physiological similarity to humans. However, differences between species create a domain shift that complicates direct model transfer from human to pig data. Recently, foundation models trained on large human datasets have shown promise for robust medical image segmentation; yet their applicability to porcine data remains largely unexplored. In this work, we investigate whether foundation models can generate sufficiently accurate pseudo-labels for pig cardiac CT and propose a simple self-training approach to iteratively refine these labels. Our method requires no manually annotated pig data, relying instead on iterative updates to improve segmentation quality. We demonstrate that this self-training process not only enhances segmentation accuracy but also smooths out temporal inconsistencies across consecutive frames. Although our results are encouraging, there remains room for improvement, for example by incorporating more sophisticated self-training strategies and by exploring additional foundation models and other cardiac imaging technologies.
Application of Machine Learning in Early Recommendation of Cardiac Resynchronization Therapy
Sep 13, 2021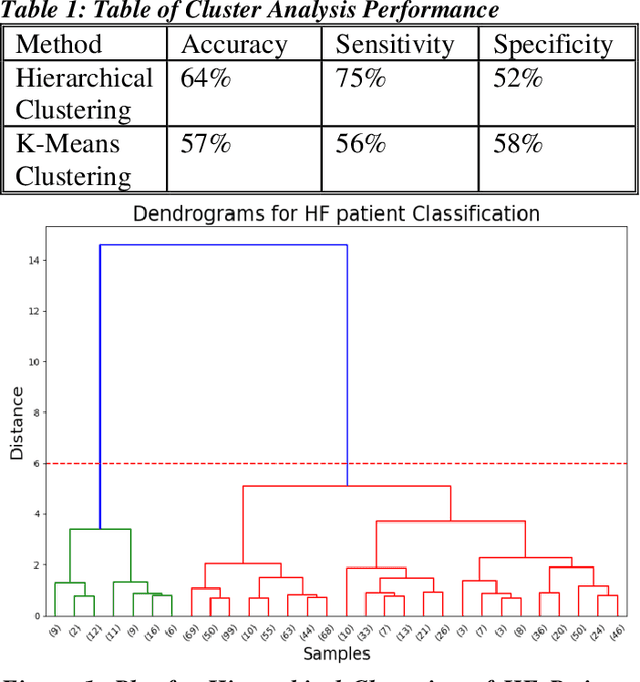
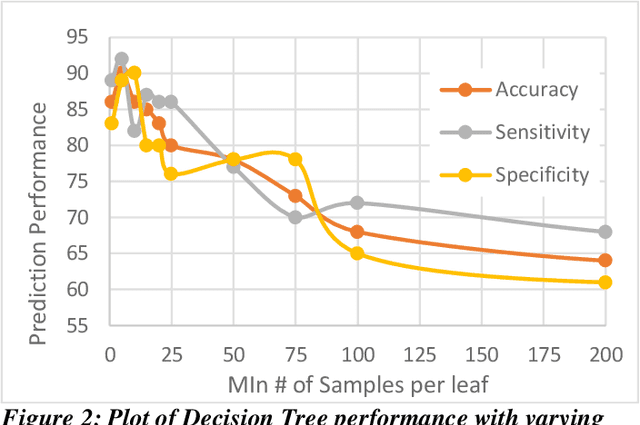
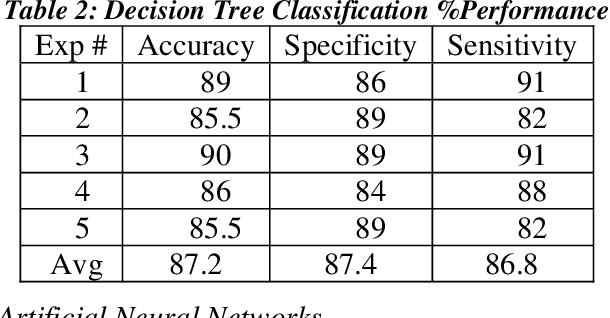
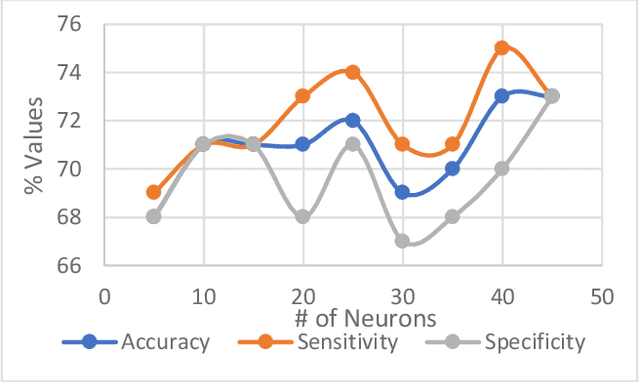
Abstract:Heart failure (HF) is a leading cause of morbidity, mortality, and health care costs. Prolonged conduction through the myocardium can occur with HF, and a device-driven approach, termed cardiac resynchronization therapy (CRT), can improve left ventricular (LV) myocardial conduction patterns. While a functional benefit of CRT has been demonstrated, a large proportion of HF patients (30-50%) receiving CRT do not show sufficient improvement. Moreover, identifying HF patients that would benefit from CRT prospectively remains a clinical challenge. Accordingly, strategies to effectively predict those HF patients that would derive a functional benefit from CRT holds great medical and socio-economic importance. Thus, we used machine learning methods of classifying HF patients, namely Cluster Analysis, Decision Trees, and Artificial neural networks, to develop predictive models of individual outcomes following CRT. Clinical, functional, and biomarker data were collected in HF patients before and following CRT. A prospective 6-month endpoint of a reduction in LV volume was defined as a CRT response. Using this approach (418 responders, 412 non-responders), each with 56 parameters, we could classify HF patients based on their response to CRT with more than 95% success. We have demonstrated that using machine learning approaches can identify HF patients with a high probability of a positive CRT response (95% accuracy), and of equal importance, identify those HF patients that would not derive a functional benefit from CRT. Developing this approach into a clinical algorithm to assist in clinical decision-making regarding the use of CRT in HF patients would potentially improve outcomes and reduce health care costs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge