Brian Hargreaves
Multimodal Feedback for Handheld Tool Guidance: Combining Wrist-Based Haptics with Augmented Reality
Jan 17, 2026Abstract:We investigate how vibrotactile wrist feedback can enhance spatial guidance for handheld tool movement in optical see-through augmented reality (AR). While AR overlays are widely used to support surgical tasks, visual occlusion, lighting conditions, and interface ambiguity can compromise precision and confidence. To address these challenges, we designed a multimodal system combining AR visuals with a custom wrist-worn haptic device delivering directional and state-based cues. A formative study with experienced surgeons and residents identified key tool maneuvers and preferences for reference mappings, guiding our cue design. In a cue identification experiment (N=21), participants accurately recognized five vibration patterns under visual load, with higher recognition for full-actuator states than spatial direction cues. In a guidance task (N=27), participants using both AR and haptics achieved significantly higher spatial precision (5.8 mm) and usability (SUS = 88.1) than those using either modality alone, despite having modest increases in task time. Participants reported that haptic cues provided reassuring confirmation and reduced cognitive effort during alignment. Our results highlight the promise of integrating wrist-based haptics into AR systems for high-precision, visually complex tasks such as surgical guidance. We discuss design implications for multimodal interfaces supporting confident, efficient tool manipulation.
EasyREG: Easy Depth-Based Markerless Registration and Tracking using Augmented Reality Device for Surgical Guidance
Apr 13, 2025Abstract:The use of Augmented Reality (AR) devices for surgical guidance has gained increasing traction in the medical field. Traditional registration methods often rely on external fiducial markers to achieve high accuracy and real-time performance. However, these markers introduce cumbersome calibration procedures and can be challenging to deploy in clinical settings. While commercial solutions have attempted real-time markerless tracking using the native RGB cameras of AR devices, their accuracy remains questionable for medical guidance, primarily due to occlusions and significant outliers between the live sensor data and the preoperative target anatomy point cloud derived from MRI or CT scans. In this work, we present a markerless framework that relies only on the depth sensor of AR devices and consists of two modules: a registration module for high-precision, outlier-robust target anatomy localization, and a tracking module for real-time pose estimation. The registration module integrates depth sensor error correction, a human-in-the-loop region filtering technique, and a robust global alignment with curvature-aware feature sampling, followed by local ICP refinement, for markerless alignment of preoperative models with patient anatomy. The tracking module employs a fast and robust registration algorithm that uses the initial pose from the registration module to estimate the target pose in real-time. We comprehensively evaluated the performance of both modules through simulation and real-world measurements. The results indicate that our markerless system achieves superior performance for registration and comparable performance for tracking to industrial solutions. The two-module design makes our system a one-stop solution for surgical procedures where the target anatomy moves or stays static during surgery.
Deep Learning Super-Resolution Enables Rapid Simultaneous Morphological and Quantitative Magnetic Resonance Imaging
Aug 07, 2018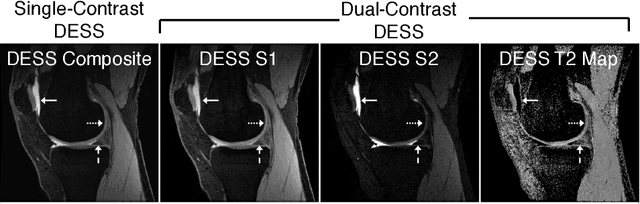
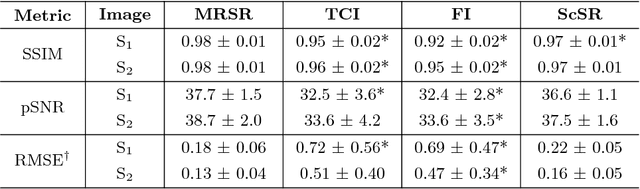
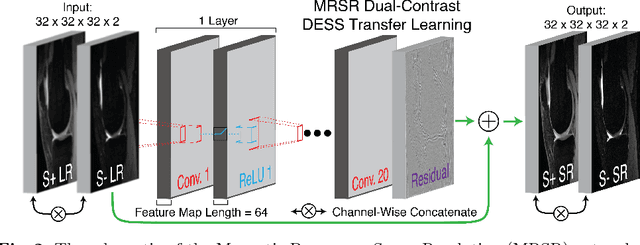
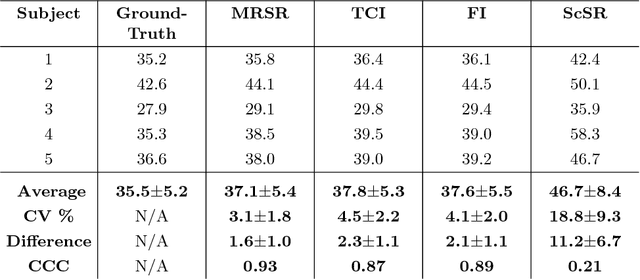
Abstract:Obtaining magnetic resonance images (MRI) with high resolution and generating quantitative image-based biomarkers for assessing tissue biochemistry is crucial in clinical and research applications. How- ever, acquiring quantitative biomarkers requires high signal-to-noise ratio (SNR), which is at odds with high-resolution in MRI, especially in a single rapid sequence. In this paper, we demonstrate how super-resolution can be utilized to maintain adequate SNR for accurate quantification of the T2 relaxation time biomarker, while simultaneously generating high- resolution images. We compare the efficacy of resolution enhancement using metrics such as peak SNR and structural similarity. We assess accuracy of cartilage T2 relaxation times by comparing against a standard reference method. Our evaluation suggests that SR can successfully maintain high-resolution and generate accurate biomarkers for accelerating MRI scans and enhancing the value of clinical and research MRI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge