Alice Nieuwboer
CARE-PD: A Multi-Site Anonymized Clinical Dataset for Parkinson's Disease Gait Assessment
Oct 05, 2025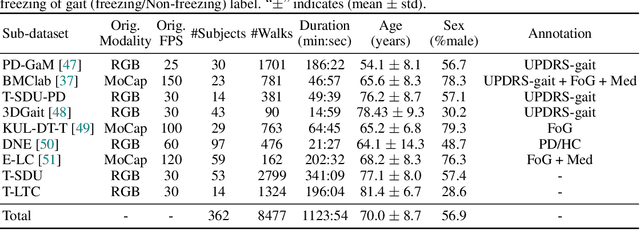
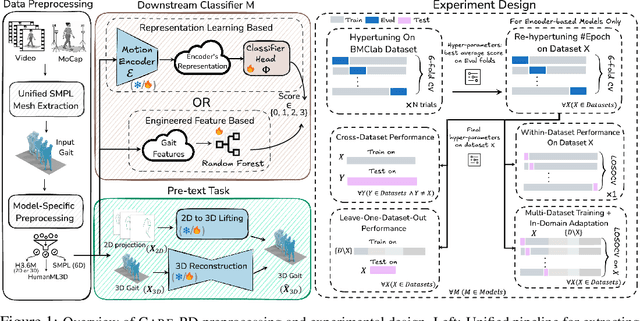
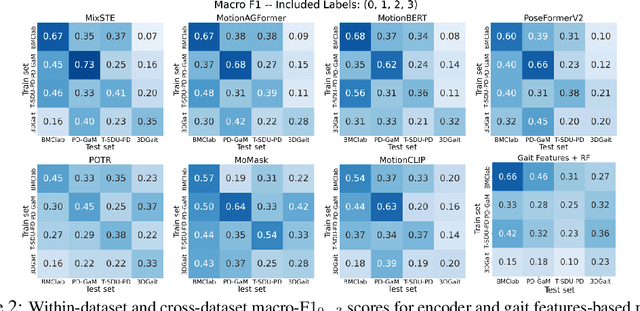
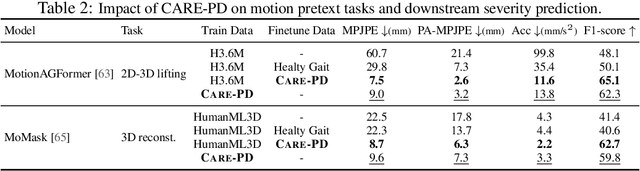
Abstract:Objective gait assessment in Parkinson's Disease (PD) is limited by the absence of large, diverse, and clinically annotated motion datasets. We introduce CARE-PD, the largest publicly available archive of 3D mesh gait data for PD, and the first multi-site collection spanning 9 cohorts from 8 clinical centers. All recordings (RGB video or motion capture) are converted into anonymized SMPL meshes via a harmonized preprocessing pipeline. CARE-PD supports two key benchmarks: supervised clinical score prediction (estimating Unified Parkinson's Disease Rating Scale, UPDRS, gait scores) and unsupervised motion pretext tasks (2D-to-3D keypoint lifting and full-body 3D reconstruction). Clinical prediction is evaluated under four generalization protocols: within-dataset, cross-dataset, leave-one-dataset-out, and multi-dataset in-domain adaptation. To assess clinical relevance, we compare state-of-the-art motion encoders with a traditional gait-feature baseline, finding that encoders consistently outperform handcrafted features. Pretraining on CARE-PD reduces MPJPE (from 60.8mm to 7.5mm) and boosts PD severity macro-F1 by 17 percentage points, underscoring the value of clinically curated, diverse training data. CARE-PD and all benchmark code are released for non-commercial research at https://neurips2025.care-pd.ca/.
Automated freezing of gait assessment with marker-based motion capture and multi-stage graph convolutional neural networks approaches expert-level detection
Apr 07, 2021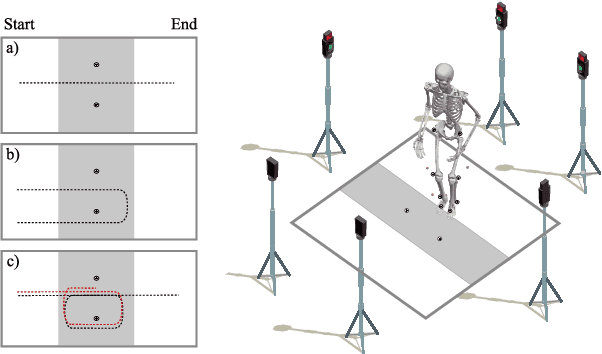
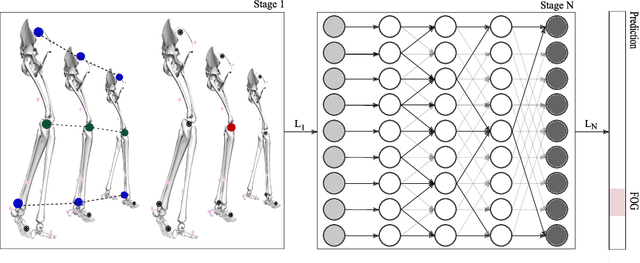
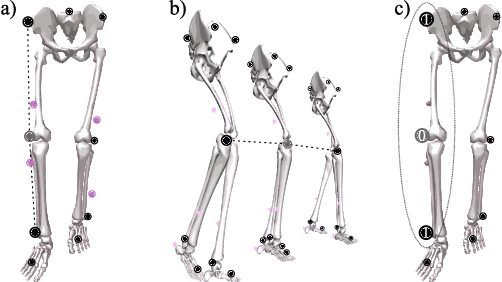
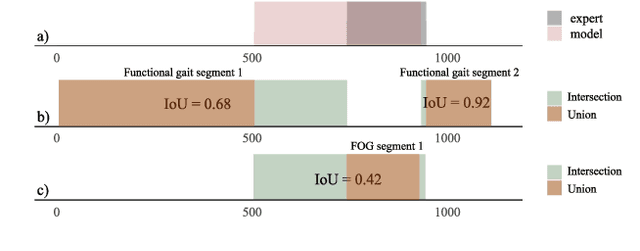
Abstract:Freezing of gait (FOG) is a common and debilitating gait impairment in Parkinson's disease. Further insight in this phenomenon is hampered by the difficulty to objectively assess FOG. To meet this clinical need, this paper proposes a motion capture-based FOG assessment method driven by a novel deep neural network. The proposed network, termed multi-stage graph convolutional network (MS-GCN), combines the spatial-temporal graph convolutional network (ST-GCN) and the multi-stage temporal convolutional network (MS-TCN). The ST-GCN captures the hierarchical motion among the optical markers inherent to motion capture, while the multi-stage component reduces over-segmentation errors by refining the predictions over multiple stages. The proposed model was validated on a dataset of fourteen freezers, fourteen non-freezers, and fourteen healthy control subjects. The experiments indicate that the proposed model outperforms state-of-the-art baselines. An in-depth quantitative and qualitative analysis demonstrates that the proposed model is able to achieve clinician-like FOG assessment. The proposed MS-GCN can provide an automated and objective alternative to labor-intensive clinician-based FOG assessment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge